
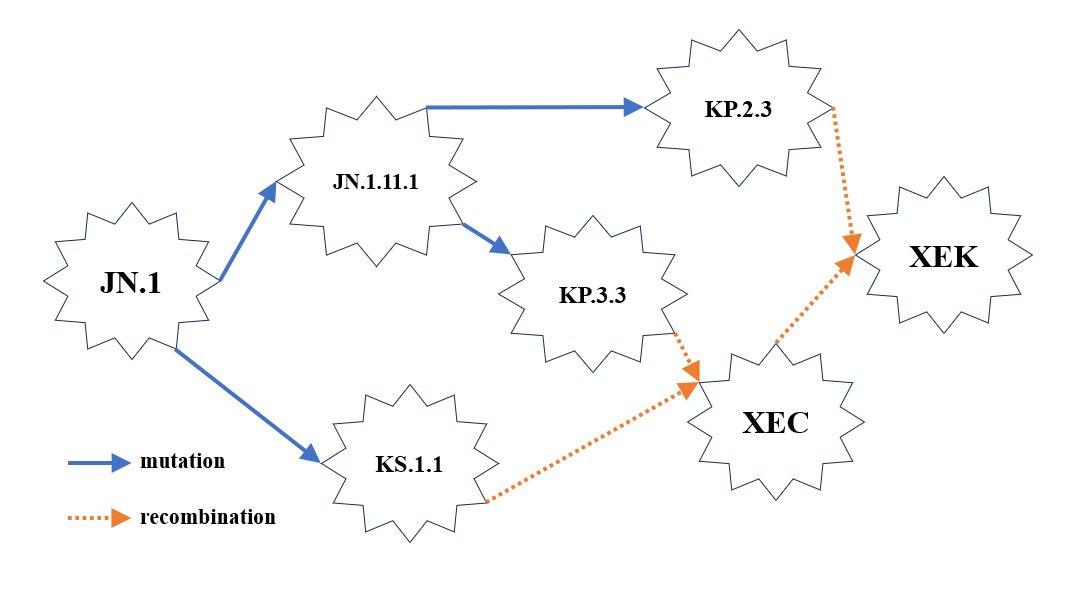
XEK ( XEC and KP.2.3 Recombinant Variant)
XEK is an XEC and KP.2.3 recombinant variant. In less than 2 months, 3 different variants were able to recombine to create one new variant, which happens to have the greatest growth advantage at this time. The first thing this tells us is that more people are being infected with more than one variant at a time. In the simplest terms, that’s very bad for your health. This is also allowing for more opportunities for the virus to evolve. The faster it evolves, the less likely prior immune responses to earlier variants, or that induced from vaccines, will be effective. It’s also making it less likely that other treatments, like Paxlovid or monoclonal antibodies, will be effective.
The CDC and the other public health agencies around the world that followed the CDC’s unscientific guidance to allow people to return to school or work after being fever-free for 24 hours when most people are still at peak levels of contagiousness, not only puts everyone’s health in jeopardy, it aids the viruses evolution, which allows it to overcome our immune system and the treatments designed to aid the immune response or slow down the virus.
Xu Zou-
https://x.com/xz_keg/status/1840242117748334978?t=OUApusQHt8IGAQRU2_Rk8A&s=19
XEK ( XEC and KP.2.3 Recombinant Variant)
XEK has been found in Austria, Croatia, Denmark, France, Germany, Ireland, Netherlands, Slovenia, Sweden, Ukraine, the United Kingdom, and the United States. Due to the delays in sequencing, we can assume that it has spread much further than this already.
What did XEK gain over XEC that could explain an advantage?
This is where it gets even more concerning. Unlike many earlier variants, there aren’t any changes in the Spike protein. This could very well be a reflection of a world with much less influence from the vaccines, which target the Spike protein. The virus didn’t have any selective pressure to alter the mutations in the spike, but it continues to have pressure from the innate immune system. A strong mucosal response and T-cell response is what is keeping many people from becoming symptomatically infected.
We discussed in the last update, how XEC may be better at avoiding these responses. It appears that XEK may have gotten just a little better at it than XEC. Keep in mind this is still an early assessment. XEC is fairly well established at this point and will very likely become dominant sometime in mid-November. XEK is gaining enough ground, now found across Europe and a few sequences in the U.S. There haven’t been any found in Canada or Australia, yet. We know that if it isn’t there now, it will be very soon.
T.A.C.T. is a reader-supported publication. To receive new posts and support this work, consider becoming a free or paid subscriber.
XEC and XEK are recombinant variants of SARS-CoV-2 that are very similar and only differ in a few nucleotide mutations and associated ORF1a proteins, which could influence the virus's fitness, transmissibility, and immune evasion capabilities. There isn't any change in the Spike protein. Here’s a detailed comparison:
1. Nucleotide Mutations:
XEC contains the mutation ORF1a: A599T.
XEK has gained two mutations in the ORF1a region:
ORF1a: H110A
ORF1a: T2283I
Additionally, XEK dropped the A599T mutation present in XEC.
XEK also features four new nucleotide mutations not found in XEC:
C593T
C3505T
T5101C
C9286T
These mutations primarily fall within the ORF1a region, which encodes for critical non-structural proteins essential for viral replication and RNA processing.
2. Potential Impact on Viral Fitness:
Mutations in the ORF1a region can significantly affect the virus's replication machinery and its ability to evade the host immune system. The presence of H110A and T2283I may enhance the virus's replication efficiency or alter its interaction with host cellular factors.
Dropping the A599T mutation could remove a potential deleterious effect, while the new mutations may confer a beneficial adaptation to the viral replication process.
3. Growth Advantage:
XEK demonstrates a modest growth advantage over XEC, attributed to its distinct combination of nucleotide mutations in the ORF1a region. These changes are believed to improve viral replication efficiency and may facilitate increased transmissibility or immune escape.
Hypothesis on Potential Impact on ORF8 and Other Proteins:
ORF8 is a protein linked to immune evasion, and its truncation can reduce the virus's ability to evade the host immune system. In certain variants, such as Δ382, truncated ORF8 was associated with decreased immune escape capabilities.
While mutations in XEK do not directly alter the coding sequence of ORF8, the changes in the ORF1a region could indirectly influence ORF8 expression. The hypothesis is that:
Dropping G2060A may affect RNA splicing or regulatory elements, potentially restoring ORF8 expression and enhancing immune evasion capabilities.
Phosphorylation or other post-translational modifications in non-structural proteins from ORF1a could also impact ORF8’s stability or function.
Other Potential Proteins Affected:
Changes in ORF1a can also affect other non-structural proteins, including:
NSP3, which is involved in viral replication and immune modulation.
NSP5 (3CLpro), a protease critical for processing viral polyproteins, which may influence overall viral replication efficiency.
NSP12, the RNA-dependent RNA polymerase, is essential for viral RNA synthesis and replication.
Conclusion:
XEC and XEK exhibit distinct sets of nucleotide mutations, particularly in the ORF1a region. In XEK, the acquisition of H110A and T2283I mutations, along with the loss of A599T, are believed to enhance viral fitness and transmissibility. It is hypothesized that changes in ORF1a may indirectly affect ORF8 truncation, potentially restoring its full-length form and thereby enhancing immune evasion. Additionally, mutations in ORF1a could influence other critical viral proteins, such as NSP3, NSP5, and NSP12, which play essential roles in the virus's life cycle and interactions with host immune responses.





Regarding the quote from Margaret Mead above, she likely never encountered a brain melting virus.
We know from autopsy studies, that SARS-CoV-2 colonizes the corpus callosum of the brain, a region of the brain involved in the assessment of risk. Curious that.
Thank you.
Jessica Wildfire wrote again about bird flu.
Is anything at all being done to track? How do we get the vaccine?
Could people get infected with sarscov2 and bird flu simultaneously? That seems like an absolute nightmare. Our demolished healthcare system can’t handle a covid wave…
We don’t have capacity for the numbers of sick people.
And since sarscov2 can cause sepsis, we will see more deaths from sepsis.
Sepsis survivor here. 🙋🏽♀️It is so painful. You feel like every cell is imploding. You need immediate attention- or you will die. Period.