COVID-19 continues to explore new avenues to infiltrate human cells, accumulating mutations as it progresses in its evolutionary journey. The pace and trajectory of this evolution are highly alarming.
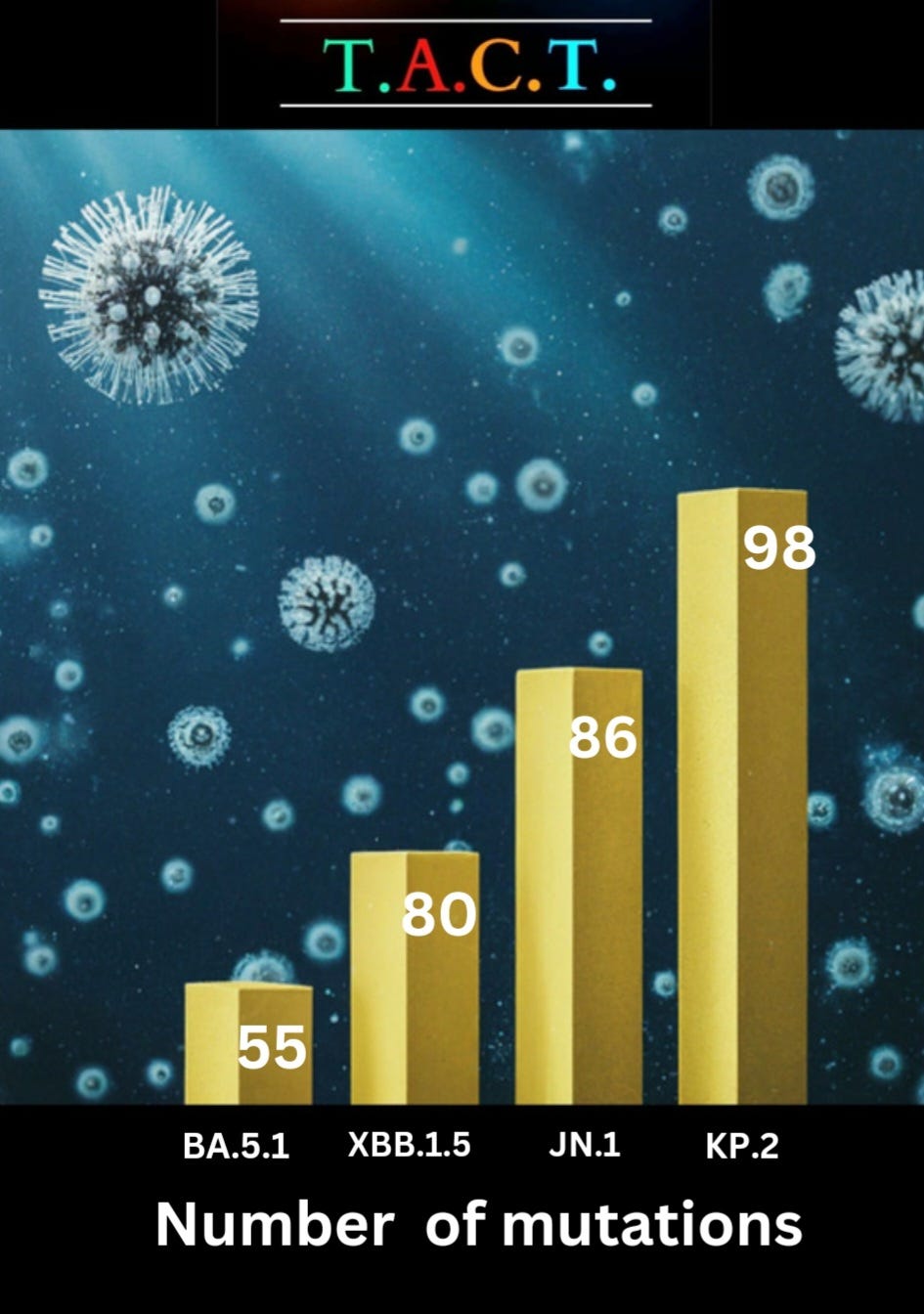
A recent study published on January 16, 2024, sheds light on some troubling developments in the earlier phases of the Omicron variant's evolution. It reveals that variants such as BA.4 and BA.5 display enhanced abilities to evade the body's natural defenses compared to predecessors like BA.1 and BA.2. This worrisome trend persists with each subsequent evolutionary leap, as seen with variants like KP.2, which boasts a staggering 45 mutations distinct from XBB.1.5. The sheer accumulation of mutations underscores a clear narrative: the virus is acquiring new capabilities as it adapts.
In simpler terms, the Omicron variants are evolving to undermine our immune system's effectiveness from more than one angle. Researchers attribute this phenomenon to heightened levels of specific viral proteins that impede our innate defense mechanisms. They suggest that this enhanced evasion of our immune system ranks as the second most influential factor driving the virus's evolution, surpassed only by its knack for evading neutralizing antibodies resulting from prior infections or vaccinations. Variants like JN.1 and now KP.2 are perpetuating this trend.
Encountering a variant that has evolved to evade our immune response can weaken the efficacy of our defenses. As XBB.1.5 becomes obsolete, variants like JN.1 emerge to sidestep the immunity conferred by XBB.1.5 vaccines and infections. KB.2 (JN.1.11.1.2) is swiftly spreading within environments where JN.1 is prevalent, allowing it to outmaneuver immune defenses mounted against JN.1.
A History of COVID’s Evolution of Immune Evasion from Vaccines and Infections
As we delve into the studies below, presented in chronological order, a distinct pattern emerges: COVID-19 consistently evolves to evade our immune responses, whether triggered by vaccines or prior infections. Research indicates that the virus is altering its spike protein to evade antibodies, signaling just the beginning of its adaptive strategies. COVID's evolution extends beyond spike proteins, potentially enabling it to circumvent additional aspects of our immune system, infect various cell types (including immune and brain cells), and establish persistent infections more readily.
These prolonged infections, where the virus persists and continually adapts within the body, impose a sustained burden on the immune system and other organs, posing significant health risks. Recognizing the potential long-term health implications of COVID's evolution should inform our approach to addressing the ongoing threat posed by the virus.
Below, you'll encounter excerpts from the referenced study followed by simplified explanations aimed at making the information accessible to a broader audience, including high school students. This effort aims to enhance understanding of the gravity of the situation and underscore the importance of taking decisive action against the virus.
"Neutralization of SARS-CoV-2 by convalescent plasma and monoclonal antibodies" (Nature Medicine, 2020) https://www.pnas.org/doi/full/10.1073/pnas.2103154118?doi=10.1073/pnas.2103154118:
This study explored the effectiveness of convalescent plasma and monoclonal antibodies in neutralizing SARS-CoV-2, highlighting potential limitations in antibody responses due to viral mutations.
Simplified: This study looked at whether treatments using antibodies from recovered COVID-19 patients (convalescent plasma) or lab-made antibodies (monoclonal antibodies) still worked against the virus. They found that the virus can change (mutate) in ways that make these antibody treatments less effective.
“Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity” (Published:March 12, 2021DOI:https://doi.org/10.1016/j.cell.2021.03.013)
“Cross-neutralization of B.1.351 variants was comparable to SARS-CoV and bat-derived WIV1-CoV, suggesting that a relatively small number of mutations can mediate potent escape from vaccine responses. While the clinical impact of neutralization resistance remains uncertain, these results highlight the potential for variants to escape from neutralizing humoral immunity and emphasize the need to develop broadly protective interventions against the evolving pandemic.”
Simplified: This study looked at a COVID-19 variant called B.1.351. It found that this variant was harder for the immune system to fight off – even for people who were vaccinated. This means that small changes in the virus can make it better at avoiding existing immunity.
“Immune Evasion of SARS-CoV-2 Emerging Variants: What Have We Learnt So Far?” (Viruses June 15, 2021, 13(7), 1192; https://doi.org/10.3390/v13071192)
“The potential to evade neutralization is the result of diversity of the target epitopes generated by the accumulation of mutations in the spike protein. While three globally recognized VOCs (Alpha or B.1.1.7, Beta or B.1.351, and Gamma or P.1) remain sensitive to neutralization albeit at reduced levels by the sera of convalescent individuals and recipients of several anti-COVID19 vaccines, the effect of spike variability is much more evident on the neutralization capacity of monoclonal antibodies. The newly recognized VOC Delta or lineage B.1.617.2, as well as locally accepted VOCs (Epsilon or B.1.427/29-US and B1.1.7 with the E484K-UK) are indicating the necessity of close monitoring of new variants on a global level.”
Simplified: If the virus changes this spiky protein too much, our bodies might have trouble recognizing and fighting it off.
This study found that some newer versions of COVID-19 (variants) like Delta, have changed their spiky protein enough to make it a little harder for our immune system to fight them, even for people who already had COVID-19 or got a vaccine. It shows the virus can change to avoid our defenses.
"Increased immune escape of the new SARS-CoV-2 variant of concern Omicron” (Cell Mol Immunol 19, 293–295 (January 2022). https://doi.org/10.1038/s41423-021-00836-z)
Consistent with other studies, they observed that protective immunity after previous infection could barely neutralize Omicron. Even worse, almost all vaccines that have been extensively used exhibited remarkably reduced neutralization against Omicron. Taken together the results suggested a higher risk of Omicron breakthrough infections and reduced efficiency of the protective immunity elicited by existing vaccines.
Simplified: Studies looked at people who already had COVID-19 and found that their immune systems didn't do a great job fighting off the Omicron variant.
Even most vaccines weren't very effective at stopping Omicron.
This means there was a much higher chance of catching Omicron even if you had COVID-19 before or received a vaccine. It also means the vaccines didn’t offer as much protection against infection with the Omicron variant.
“Differential neutralizing antibody responses elicited by CoronaVac and BNT162b2 against SARS-CoV-2 Lambda in Chile” (Nature Microbiology volume 7, pages 524–529 (April 2022) https://doi.org/10.1038/s41564-022-01092-1
“The data presented here also show that the unique pattern of mutations present in the spike protein of VOI Lambda confers escape from neutralizing antibodies elicited by CoronaVac, BNT162b2 and convalescent plasma, which is in agreement with recent observations25. The escape by Lambda from neutralizing antibodies was higher than that observed for Alpha and Gamma variants, but lower than that observed for Delta, indicating that escape from neutralizing antibodies is a key feature of this globally dominant variant.”
Simplified: This study looked at a COVID-19 variant called Lambda. They found that Lambda was better at dodging antibodies from people who were vaccinated with certain vaccines (like CoronaVac and BNT162b2), and even people who had recovered from an earlier COVID infection.
Lambda wasn't quite as good at dodging antibodies as another variant called Delta, but it was better than some others (Alpha and Gamma). Overall, this shows that the virus can change in ways that help it avoid our immune system's defenses.
"SARS-CoV-2 Omicron BA.1 escapes neutralization by most COVID-19 vaccines and natural infections" (Nature, July 7, 2022)https://www.nature.com/articles/s41564-022-01143-7:
This study demonstrated the significant immune escape of the Omicron BA.1 subvariant, raising concerns about vaccine efficacy and potential reinfections.
Simplified: This study looked at the first Omicron variant called BA.1. They found that it's really good at evading our immune system's defenses. This is a problem because it means vaccines don’t work as well against it, and as we found out, people who already had COVID-19 could get reinfected with this variant.
“Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster” (Nature Medicine, Published: 06 December 2022: https://www.nature.com/articles/s41591-022-02162-x
"The results showed that a BA.5 bivalent booster elicited a high neutralizing titer against BA.4/5 measured at 14–32 days after boost; however, the BA.5 bivalent booster did not produce robust neutralization against the newly emerged BA.2.75.2, BQ.1.1 or XBB.1.”
Simplified: Scientists studied a new booster shot designed to fight the BA.5 version of COVID-19. They found it worked really well against the BA.4 and BA.5 versions as intended. However, the booster didn't work as well against some new variations of the virus. (BA.2.75.2, BQ.1.1) They found that it barely worked at all against the XBB variants.
“Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution” ( Nature, Published: 19 December 2022: https://www.nature.com/articles/s41586-022-05644-7
”Strikingly, the BJ.1/BM.1.1.1 recombinant strains XBB and XBB.1 (XBB+G252V) are among the most humoral immune evasive strains tested, comparable to those of CH.1.1, BQ.1.1.10, and BA.4.6.3.” “These results suggest that current herd immunity and BA.5 vaccine boosters may not efficiently prevent the infection of Omicron convergent variants.”
Simplified: Scientists found new versions of the Omicron variant called XBB and XBB.1 that are extremely good at dodging our body's immune defenses. They're among the best at this out of all the variants they tested. This means that even if lots of people have already been sick with COVID-19 or received older vaccines (like the BA.5 booster), these new variants were still able to infect people. Do you see the trend yet?
"SARS-CoV-2 variant biology: immune escape, transmission and fitness" (Nat Rev Microbiol 21, 162–177 (January 18, 2023). https://doi.org/10.1038/s41579-022-00841-7)
“We hypothesize that the capacity of SARS-CoV-2 to spread efficiently is strongly linked to its ability to evade and antagonize innate immune responses in the first cells that encounter the virus in the airway.” “Although our understanding of SARS-CoV-2 is improving, virus evolution is inherently unpredictable, and a likely future scenario is the emergence of a new VOC that is antigenically and, potentially, phenotypically distinct from the early forms of Omicron.”
Simplified: These researchers think COVID-19 spreads so easily because it's very good at hiding from and fighting off our body's natural defenses (especially in the nose and throat where the virus first enters). The virus keeps changing in ways we can't predict. It's possible that a completely new version of the virus could pop up, one that's so different from our current vaccines, that past immunity won't offer much protection.
“Transmissibility, infectivity, and immune resistance of the SARS-CoV-2 BA.2.86 variant” (September 07, 2023; https://www.biorxiv.org/content/10.1101/2023.09.07.556636v1 )
“The sera obtained from individuals vaccinated with 3rd-dose monovalent, 4th-dose monovalent, BA.1 bivalent, and BA.5 bivalent mRNA vaccines exhibited very little or no antiviral effects against BA.2.86. Moreover, the three monoclonal antibodies (Bebtelovimab, Sotrovimab and Cilgavimab), which worked against the parental BA.2, did not exhibit antiviral effects against BA.2.86. These results suggest that BA.2.86 is one of the most highly immune evasive variants ever.”
Simplified: Scientists tested blood samples from people who received different COVID-19 vaccines (including multiple doses and updated boosters). They found that these vaccines didn't work very well against a new variant called BA.2.86. They also tested special treatments (antibodies) that used to work against earlier versions of COVID-19. These treatments didn't work against BA.2.86 either. This means BA.2.86 is really good at dodging our immune defenses, even better than some other variants we've seen before.
"Effectiveness of a fourth SARS-CoV-2 vaccine dose in previously infected individuals from Austria" (First published: 30 November 2023: https://doi.org/10.1111/eci.14136 / https://onlinelibrary.wiley.com/doi/10.1111/eci.14136
“In previously infected individuals, a fourth vaccination was not associated with COVID-19 death risk, but with transiently reduced risk of SARS-CoV-2 infections and reversal of this effect in longer follow-up.”
Simplified: This study looked at people who already had COVID-19 and got a fourth dose of the vaccine. They found that the fourth dose didn't seem to make a big difference in how likely they were to get seriously sick or die from COVID-19. However, it did offer some short-term protection from getting infected again but this protection didn't last very long. (2 to 3 months) Overall, this suggests that for people who've already had COVID-19, a fourth dose might not be a huge benefit.
“Fast evolution of SARS-CoV-2 BA.2.86 to JN.1 under heavy immune pressure” (published December 15, 2023) DOI:https://doi.org/10.1016/S1473-3099(23)00744-2
“In summary, JN.1, by inheriting BA.2.86's antigenic diversity and acquisition of L455S, rapidly achieved extensive resistance across receptor binding domain class 1, 2, and 3 antibodies,1 and showed higher immune evasion compared with BA.2.86 and other resistant strains like HV.1 and JD.1·1, at the expense of reduced human ACE2 binding. This evolutionary pattern, similar to the previous transition from BA.2.75 to CH.1.1 and XBB,( 2, 3, 9 ) highlights the importance of closely monitoring strains with high human ACE2 binding affinity and distinct antigenicity, like BA.2.86 and BA.2.75” “They have the potential to quickly accumulate highly immune-evasive mutations”
Simplified: JN.1 is a new variant of COVID-19 that is the offspring or child of another variant called BA.2.86. It has a mutation (L455S) that makes it really good at dodging our immune system's defenses, even more so than BA.2.86 and some other variants. Scientists are worried about variants like BA.2.86 and BA.2.75 because they can easily change further and become even better at avoiding our immunity. That's why scientists need to keep a close eye on these kinds of variants.
“Virological characteristics of the SARS-CoV-2 JN.1 variant” (published January 3, 2024) https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(23)00813-7/fulltext
“JN.1 shows robust resistance to monovalent XBB.1.5 vaccine sera compared with BA.2.86 (appendix 17–18). Taken together, these results suggest that JN.1 is one of the most immune-evading variants to date. Our results suggest that Leu455Ser contributes to increased immune evasion, which partly explains the increased reproductive number of JN.1.”
Simplified: Scientists studied people who got vaccinated with a specific vaccine (XBB.1.5) and looked at how their immune systems responded to the JN.1 variant. They found that the antibodies from the vaccine were much less effective against JN.1 compared to another variant, BA.2.86. Basically, JN.1 is very good at dodging the immune system's defenses, even from people who got vaccinated. This makes JN.1 one of the most evasive variants we've seen so far. The study also suggests that a specific change (Leu455Ser) in the virus is a big reason why JN.1 can avoid our immunity so well. This change might also be why JN.1 spreads more easily than other variants.
“Antiviral humoral immunity against SARS-CoV-2 omicron subvariants induced by XBB.1.5 monovalent vaccine in infection-naive and XBB-infected individuals” (Published: January 08, 2024)
DOI:https://doi.org/10.1016/S1473-3099(23)00784-3
“These observations suggest that a single dose of XBB.1.5 monovalent vaccine potentially induces antiviral humoral immunity against XBB subvariants as well as BA.2.86 without previous infection.” “It should also be noted that the postvaccination sera were collected 20–29 days after vaccination, and it is possible that the immunity is mature at these later time points.”
Simplified: The study looked at people who got a single dose of the XBB.1.5 targeted vaccine. The scientists wanted to see if the vaccine helped against that variant and similar ones (like BA.2.86). They found that the vaccine seemed to work against XBB and its descendants, but wasn’t clear on BA.2.86. They did not look at JN.1.
“Immune evasion, infectivity, and fusogenicity of SARS-CoV-2 BA.2.86 and FLip variants” (February 1, 2024) https://www.cell.com/cell/pdf/S0092-8674(23)01400-9.pdf
“BA.2.86 presents distinct biology from BA.2 and XBB variants.” “We find that BA.2.86 displays decreased infectivity in 293T-ACE2 cells not only compared to the ancestral BA.2/BA.1 but also relative to more recent XBB variants, including XBB.1.5.” “Strikingly, in CaLu-3 cells, BA.2.86 exhibits a higher infectivity as well as enhanced cell-cell fusion compared to the ancestral BA.2 and some XBB variants. These results suggest that the spike protein of BA.2.86 may be more conformational stable compared to the parental BA.2 and XBB variants.”
“The increased infectivity of BA.2.86 in CaLu-3 cells is somewhat alarming. CaLu-3 represents a biologically relevant cell line that is derived from human lung epithelia type II pneumo-cytes and is known to express endogenous levels of ACE2 and host co-factor TMPRSS2, the latter of which is critical for the respiratory tract tropism for SARS-CoV-2.4,47–49 It has been established that CaLu-3 cells are almost exclusively infected through the TMPRSS2-reliant plasma membrane fusion pathway, while the endosomal pathway is used in 293T-ACE2 cells due to the lack of TMPRSS2. Furthermore, comparisons between the Delta and Omicron variants demonstrated that Omicron associates with increased transmissibility but decreased pathogenicity versus Delta. 2–4 Our data shown here suggest that BA.2.86 may have an increased tendency of using the plasma membrane route of entry as opposed to the endosomal route of entry. Our molecular modeling suggests that mutations present in BA.2.86 and XBB variants can alter the spike binding to ACE2 receptor, therefore impacting membrane fusion and entry of different target cells.”
Simplified: BA.2.86 is a unique version of the COVID-19 virus - its biology makes it different from both the early Omicron strain (BA.2) and other recent variants like XBB. In some human cells (293T-ACE2), BA.2.86 isn't as good at infecting compared to both older and newer variants. But in other cells (CaLu-3 cells, found in the lungs), BA.2.86 is actually better at infecting and spreading than those other variants. Scientists believe this has to do with how the BA.2.86 spike protein is shaped. It might be more stable than the spike proteins in other variants. This finding is concerning because the way BA.2.86 infects lung cells might be similar to how more dangerous variants like Delta entered cells. This shows that COVID-19 continues to evolve in new ways that could eventually cause more severe symptoms or infect more parts of the body while continually getting better at evading the immune system making it more likely to become a persistent infection. Persistent infections lead to long-term damage and potentially debilitating symptoms.
“Protection of natural infection against reinfection with SARS-CoV-2 JN.1 variant” (published 23 February 2024) https://www.medrxiv.org/content/10.1101/2024.02.22.24303193v1
“The findings show that the protection of natural infection against reinfection with JN.1 is strong only among those who were infected within the last 6 months, with variants such as XBB*. However, this protection wanes rapidly and is entirely lost one year after the previous infection. The findings support considerable immune evasion by JN.1.”
Simplified: This study found that getting infected with an XBB variant only offers strong protection against getting reinfected with the JN.1 variant for about 6 months. After a year, that protection is completely gone. This shows that the JN.1 variant is very good at dodging the immune system's defenses. It also tells us that people infected in September, October, November, and December of 2023, will start becoming susceptible to reinfection soon and over the next few months.
“Ongoing evolution of SARS-CoV-2 drives escape from mRNA vaccine-induced humoral immunity” (published 07 March 2024) doi: https://doi.org/10.1101/2024.03.05.24303815
“Progressive loss of neutralization was observed across newer variants, irrespective of vaccine doses. Importantly, an updated XBB.1.5 booster significantly increased titers against newer variants but not JN.1. These findings demonstrate that seasonal boosters improve titers against contemporaneous strains, but novel variants continue to evade updated mRNA vaccines”
Simplified: This study found that the newer versions of COVID-19 are getting better at dodging our immune systems, even after people have been vaccinated multiple times. An updated booster shot (designed for the XBB.1.5 variant) helped boost protection against some of these newer versions of the virus, but it didn't work very well against a specific variant called JN.1. This means that even with updated vaccines, new versions of the virus are still finding ways to slip past our defenses.
“Distinct Patterns of SARS-CoV-2 BA.2.87.1 and JN.1 Variants in Immune Evasion, Antigenicity and Cell-Cell Fusion” (published March 11, 2024) doi: https://doi.org/10.1101/2024.03.11.583978
“Importantly, BA.2.87.1 exhibits higher levels of infectivity, cell-cell fusion activity, and furin cleavage efficiency than BA.2.86/JN.1. Antigenically, we found that BA.2.87.1 is closer to the ancestral BA.2 compared to other recently emerged Omicron subvariants including BA.2.86/JN.1 and XBB.1.5. Altogether, these results highlight immune escape properties as well as biology of new variants and underscore the importance of continuous surveillance and informed decision-making in the development of effective vaccines.”
Simplified: This study focused on a variant called BA.2.87.1. It found that this variant is better at infecting cells and spreading within the body than some other Omicron subvariants (like BA.2.86/JN.1). Interestingly, even though BA.2.87.1 has changed a lot, it's more similar to the early Omicron strain (BA.2) in terms of how the immune system sees it. These findings show that the virus keeps evolving ways to trick our immune system. They also highlight the importance of scientists tracking new variants. As highlighted in the #12 study on this list, a variant like BA.2.87.1 can quickly accumulate new mutations, giving it a much greater advantage.
“Incidence, risk factors, and clinical symptom profile of reinfection during Omicron-dominated COVID-19 outbreak in Hong Kong: A retrospective cohort study” (March 15, 2024) https://www.medrxiv.org/content/10.1101/2024.03.12.24303945v1 )
"No significant difference in mRNA (BNT162b2) vaccine and inactivated (CoronaVac) vaccine against reinfection."
Simplified: This study looked at two different COVID-19 vaccines (Pfizer and CoronaVac) and whether they affected your chances of getting re-infected. They found that there wasn't much difference between the two vaccines in preventing reinfection. 19. "KP.2 shows the most significant resistance to the sera of monovalent XBB.1.5 vaccinee without infection (3.1-fold) as well as those who with infection (1.8-fold). Altogether, these results suggest that the increased immune resistance ability of KP.2 partially contributes to the higher Re more than previous variants including JN.1." (May 20, 2024)
"Virological characteristics of the SARS-CoV-2 KP.2 variant"
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(24)00298-6/fulltext

The pattern is unmistakable: with each passing year since the cessation of mitigation measures, the evolution of COVID-19 accelerates. It's highly probable that from 2025 to 2026, we'll witness a continual rise in the proliferation of variants, accompanied by a surge in mutations aimed at evading our immune defenses, thereby extending the virus's ability to persist in our bodies. Please share any questions or insights you may have.





The Cleveland Clinic study didn’t show causation for risks going up after the booster. For any later studies it would make sense for boosted people to be at higher risk after 3 months because only higher risk people are getting boosted at this point.
And if it is a concern for MRNA, it’s not a concern for Novavax, which is the superior vaccine from what I’ve read.