Several studies in January have shown Paxlovid's inability to prevent Long COVID and a new study published in the New England Journal of Medicine on April 3, 2024, casts further doubt on Paxlovid's efficacy.
Key Findings
Nirmatrelvir–ritonavir (the active ingredients in Paxlovid) was not associated with a significantly shorter time to sustained alleviation of COVID-19 symptoms compared to a placebo.
The usefulness of nirmatrelvir–ritonavir in patients who are not at high risk for severe COVID-19 has not been established.
These findings suggest that Paxlovid may not be as effective as previously thought, particularly for individuals not considered high-risk for severe illness.
The study raises questions about the drug's overall efficacy and its role in managing COVID-19 going forward.
"Nirmatrelvir–ritonavir was not associated with a significantly shorter time to sustained alleviation of Covid-19 symptoms than placebo, and the usefulness of nirmatrelvir–ritonavir in patients who are not at high risk for severe Covid-19 has not been established."
https://www.nejm.org/doi/full/10.1056/NEJMoa2309003
Other Paxlovid Studies:
Rapid COVID-19 rebound in a severe COVID-19 patient during a 20-day course of Paxlovid (Aug 19, 2022)
https://www.journalofinfection.com/article/S0163-4453(22)00478-9/fulltext
Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomized controlled study" (April 2023)
“Paxlovid showed no significant reduction in the risk of all-cause mortality on day 28 and the duration of SARS–CoV-2 RNA clearance in hospitalized adult COVID-19 patients with severe comorbidities."
https://www.thelancet.com/journals/lanwpc/article/PIIS2666-6065(23)00012-3/fulltext
"Azvudine versus paxlovid for oral treatment of COVID-19 in Chinese patients" (Jan 3, 2024)
"Among adults who were hospitalized with SARS-CoV-2 infection, azvudine was non-inferior to paxlovid in terms of time to sustained clinical recovery, death rates, hospitalization time and cost, with few safety concerns."
https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-023-08828-2
"Exploring Paxlovid Efficacy in COVID-19 Patients with MAFLD: Insights from a Single-Center Prospective Cohort Study" (Jan 12 2024)
Abstract: “This study investigates Metabolic-associated Fatty Liver Disease (MAFLD) and COVID-19, exploring the impact of MAFLD on disease severity, outcomes, and the efficacy of the antiviral agent Paxlovid (nirmatrelvir/ritonavir). MAFLD, affects a quarter of the world's population.”
Conclusion: "Paxlovid, in patients with MAFLD and COVID-19. Although no significant distinctions were observed in hospitalization duration, oxygen saturation, or severity based on MAFLD status, Paxlovid treatment correlated with reduced hospitalization duration and improved oxygen saturation at discharge, regardless of MAFLD presence."
https://www.mdpi.com/1999-4915/16/1/112




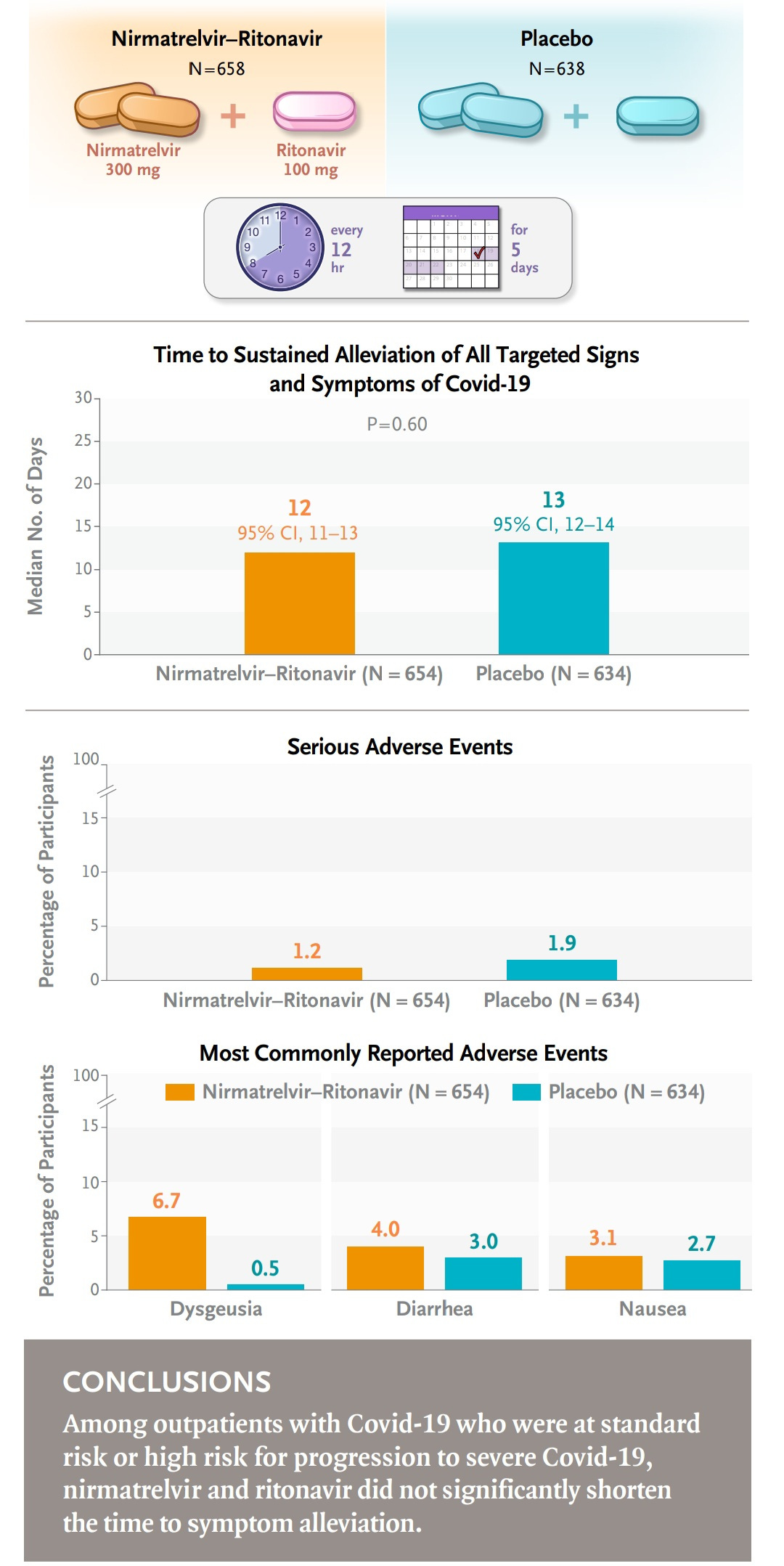
...and the remarkable thing is, this study was funded by Pfizer acknowledging their drug doesn't work.
Reminds me of that meme with the two astronauts floating in space.
Astronaut 1: "You mean Paxlovid doesn't work?"
Astronaut 2: "Never did."
Now, on to something completely different if you haven't seen it.
The reference linked below is written in a very odd way.
The TITLE seems to promote transitioning to annual SARS-CoV-2 vaccination, but the rest of the abstract doesn't appear to address the pros/cons of yearly vaccination, but rather best practices in dosing regimens.
Anyway, thought you might find it of interest...
https://www.acpjournals.org/doi/10.7326/M23-2451