How COVID Hijacks Immune Cells in the Lungs and an Immune Response Found to Reduce Hospitalizations and Deaths
Viral Entry Mechanisms, Potential Treatment Approach, Mutations that Could Evade the Immune Response
A shocking new study reveals how the COVID-19 virus ruthlessly hijacks a specific type of immune cell in the lungs, dominating its functions and triggering a dangerous cascade of events. This groundbreaking research sheds light on why COVID-19 can be so severe, and even in less severe cases, causes significant damage to the lungs.
The virus targets a specific type of immune cell in the lungs known as activated interstitial macrophages (IMs). These IMs, once infected, undergo a dramatic transformation, with the virus taking over more than 60% of their normal functions. This revelation not only deepens our understanding of the virus's pathogenesis but also raises important questions about potential treatment approaches.
In a separate (pre-print) study published on April 03, 2024, researchers examined how specific mutations in the spike protein's receptor-binding domain (RBD) affect the immune response to different SARS-CoV-2 strains. They identified key epitopes that played a crucial role in lowering COVID-19 mortality rates after the emergence of the Omicron variant. However, the study also highlighted the vulnerability of these epitopes to mutations.
These findings underscore the dynamic nature of the COVID-19 landscape and the importance of staying informed and empowered in the face of evolving threats. As we navigate the complexities of viral pathogenesis and immune response, independent coverage becomes invaluable in deciphering the latest research and its implications for public health.
Stay Informed, Stay Empowered: Independent Coverage
By subscribing, you'll gain access to:
Unbiased reporting: We cut through the noise and deliver critical information you need to make informed decisions about your health.
In-depth analysis: TACT goes beyond headlines to uncover details and the true impact of COVID, Bird Flu, Monkeypox, and other emerging or evolving diseases.
Empowering insights: We translate complex scientific data into actionable knowledge, putting you in control of your well-being.
Support Independent Journalism - Subscribe Today!
Don't miss out on valuable insights! Join our community of informed readers for free today. As a free subscriber, you will get an email granting you access to the full article within a few days. You will have access to earlier articles immediately.
Immediate access is available to paid subscribers. Your continued support means the world to us.
Keep in mind that this delay could be eliminated for free subscribers with just 120 more paid subscribers. Help us make this accessible without delay for everyone by becoming a paid subscriber today.
A study published on April 10, 2024, found that SARS-CoV-2, the virus that causes COVID-19, primarily infects a specific type of immune cell in the lungs called activated interstitial macrophages (IMs). These macrophages can accumulate thousands of copies of the viral genetic material (RNA) within themselves, taking over a significant portion (over 60%) of the cell's normal functions and activities.
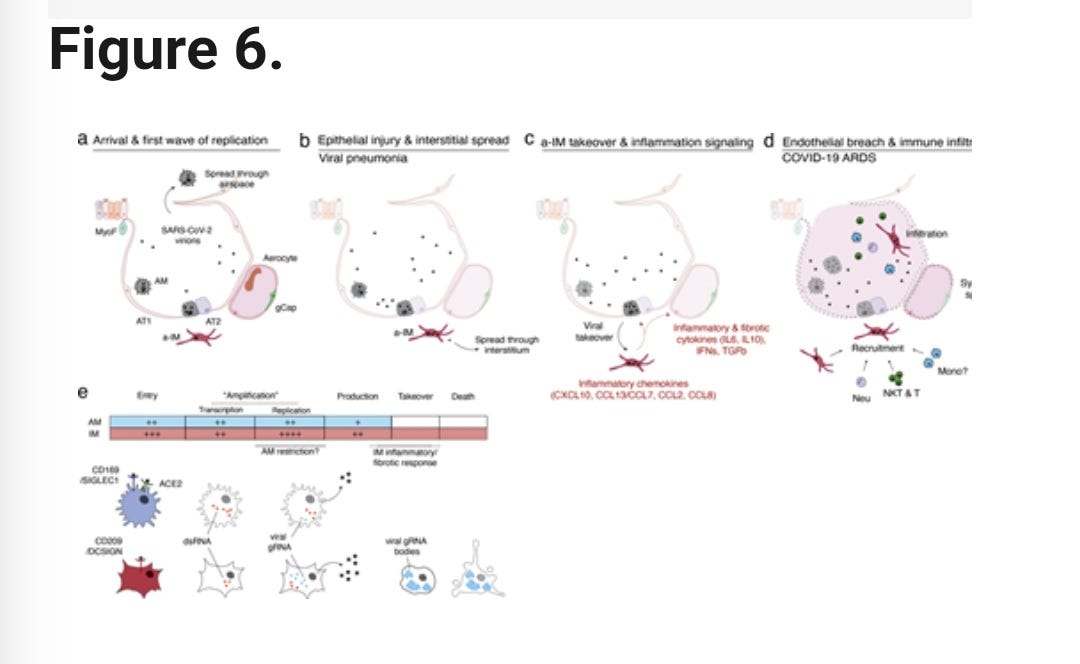
The virus also causes these infected macrophages to form dense, concentrated clusters of the viral RNA. This takeover of the cell's normal processes and the formation of these viral RNA bodies disrupt the normal architecture and structure of the host (human) cells.
In addition, the SARS-CoV-2 infection triggers the macrophages to activate two key biological programs:
Profibrotic program: This program stimulates the production of proteins involved in tissue scarring and fibrosis, such as TGF-β1 and SPP1.
Inflammatory program: This program activates an early immune response, including the release of signaling molecules like interferons, chemokines (CCL2/7/8/13, CXCL10), and inflammatory cytokines (IL-6, IL-10).
The combination of the virus taking over the host cells, the activation of profibrotic and inflammatory programs, and the disruption of normal cell architecture all contribute to the significant impact of SARS-CoV-2 infection on the human body and the development of COVID-19 disease.
Viral Entry Mechanisms
The virus can enter alveolar macrophages (AMs) using the ACE2 receptor and the Sialoadhesin/CD169 protein.
The virus can also enter interstitial macrophages (IMs) using the DC-SIGN/CD209 protein.
Implications for COVID-19 Pathology
The interstitial macrophages (IMs) appear to be a prominent site where the virus can take over the host cells, leading to inflammation and fibrosis (scarring).
This suggests that the IMs are a key focus of the viral infection and the resulting pathological processes in COVID-19 pneumonia.
Potential Treatment Approach
Targeting the CD209 (DC-SIGN) protein could be a way to prevent the early stages of the pathological processes in COVID-19 pneumonia. By blocking the virus's ability to enter the IMs through this receptor, it may be possible to disrupt the viral takeover of these cells and the subsequent inflammation and fibrosis. Importantly, the authors note that this approach of targeting viral entry pathways could be "generalized to any human lung infection." This implies that understanding the various mechanisms of viral entry into different cell types could lead to the development of broader therapeutic strategies, not just for COVID-19 but for other respiratory infections as well.
Link to Study: https://rupress.org/jem/article/221/6/e20232192/276693/Interstitial-macrophages-are-a-focus-of-viral?searchresult=1
Immune Response Found To Reduce Hospitalizations and Deaths
A (pre-print) study published April 03, 2024, looked at how 305 different HLA class I molecules (a type of protein on the surface of our cells) from over 28,000 individuals to see if they can bind to 821 peptides (small protein fragments) from the spike protein's receptor-binding domain (RBD) of 5 major SARS-CoV-2 strains. The RBD is a key part of the spike protein that the virus uses to bind to and infect our cells.
The researchers found that 4 specific mutations in the RBD 496-513 region of the Omicron B.1.1.529 variant led to a significant increase in the release of two common HLA class I epitopes (parts of the protein that the immune system can recognize). These epitopes are like flags that the immune system can use to identify and target the virus.
By analyzing global HLA class I data, the researchers discovered that populations with more of these specific HLA haplotypes (combinations of HLA proteins) had lower COVID-19 mortality rates after December 2021, when Omicron became the dominant strain. This suggests that these epitopes helped the immune system better recognize and fight off the Omicron variant.
Importantly, the newer BA.2.86 and JN.1 lineages of SARS-CoV-2 do not have any amino acid changes in the RBD 496-513 region, meaning they preserve the same core epitopes that the immune system was able to recognize with Omicron. Although the study didn’t look at KP.2, it does hold true with the newly dominant KP.2 variant. We don’t know what other methods KP.2 may have developed to evade or infect the immune system through the 7 additional ORF1a mutations, and 5 additional spike mutations over JN.1, but as far as the discussion presented in this study, it is likely to remain intact.
Could new substitutions or new mutations in S: 496 - 513 potentially jeopardize that immune-targeted response?
The study found that 4 specific mutations in the RBD 496-513 region of the Omicron B.1.1.529 variant led to a significant increase in the release of two common HLA class I epitopes. These epitopes are recognized by the immune system and helped lower COVID-19 mortality rates after Omicron became dominant. Importantly, the newer BA.2.86 and JN.1 lineages of SARS-CoV-2 do not have any amino acid changes in the RBD 496-513 region, meaning they preserve the same core epitopes that the immune system was able to recognize with Omicron.
The study says that as long as new SARS-CoV-2 variants do not acquire mutations in the critical RBD 496-513 region, they are likely to preserve the same immune-targeted epitopes that were effective against Omicron.
Any new substitutions or mutations in this specific region of the spike protein could potentially jeopardize the immune response that was effective against Omicron. Monitoring for changes in this RBD 496-513 region will be important for predicting how future variants may evade this existing immune response.
Link to the study: https://medrxiv.org/cgi/content/short/2024.04.03.24305074
Good News on Upgrading Indoor Air Quality
On April 10, 2024, The Advanced Research Projects Agency for Health (ARPA-H) announced the launch of the Building Resilient Environments for Air and Total Health (BREATHE) program, which is a platform to improve indoor air quality across the United States.
ARPA-H is a funding agency that supports research that could result in biomedical and health breakthroughs. ARPA-H's creation was proposed by the Biden administration and its establishment within the Department of Health and Human Services was approved by Congress and signed into law in March 2022.
The primary objective is to develop scalable smart building systems capable of continuous monitoring and real-time intervention to ensure safer indoor environments nationwide. This initiative is particularly significant considering the pervasive health risks associated with poor indoor air quality, which often leads to preventable respiratory illnesses such as the flu, RSV, and COVID-19. The announcement only mentioned the flu and other respiratory illnesses. They did state, “Airborne disease-causing pathogens and allergens can have a significant effect on people’s health, especially children, the elderly, and those living with chronic illnesses or are otherwise susceptible.”
One of the core focuses of BREATHE is to address the presence of airborne pathogens and allergens, which pose significant health threats, especially to vulnerable populations such as children, the elderly, and individuals with chronic illnesses. By harnessing advanced technology and data-driven approaches, the program seeks to enhance our ability to detect, assess, and mitigate these indoor air contaminants.
Key areas of research within the BREATHE program include:
Development of indoor air biosensors capable of rapidly detecting airborne biothreats
Creation of respiratory risk assessment software to evaluate potential health impacts
Optimization of building controls to enhance both health and energy efficiency
To accomplish these ambitious goals, BREATHE will collaborate with experts from various domains, including molecular diagnostics, data analysis, property management, and healthcare. By leveraging the collective expertise of these diverse stakeholders, the program aims to catalyze innovation in indoor air quality management and contribute to significant advancements in public health.
Ultimately, the success of BREATHE hinges on its ability to translate cutting-edge research into practical solutions that can be implemented on a large scale. Through the forthcoming Program Solicitation, BREATHE invites proposals from qualified teams to tackle these critical challenges and drive forward the development of next-generation building systems for safer and healthier indoor environments. Multiple awards are anticipated under the program, reflecting the importance of this initiative in addressing a pressing public health need.
"Even though Americans spend 90% of their lives indoors, we do more to monitor and reduce health threats from the air we breathe outside than we do inside," remarked BREATHE Program Manager Jessica Green. "As we experienced through the pandemic, having the ability to monitor, track, and improve the air we breathe indoors is urgently needed. BREATHE aims to revolutionize public health by transforming our ability to eliminate indoor air threats."
Proposers' Day
Hybrid Proposers' Day: May 2, 2024, 8:30 AM PST - 5:00 PM PST in Oakland, Calif.
Proposers' Day registration closes on April 30, 2024.
BREATHE Teaming Profiles
“If either you or your organization are interested in teaming, please submit your information via the form below. Your details will then be added to the list below, which is publicly available.”
Link: BREATHE Teaming Profile Form
“BREATHE anticipates that teaming will be necessary to achieve the goals of the program. Prospective performers are encouraged (but not required) to form teams with varied technical expertise to submit a proposal to the BREATHE solicitation.”
Please share your thoughts, insights, and ideas by leaving a comment.






