COVID Variant Update: The New XBB.1.16 Surging in India has Infiltrated the U.S., the U.K. and Other Countries. Why This is Concerning ("Innate Immunosuppression by ORF9b")
How ORF9B Helps COVID Suppress the Immune Response
Updated: March 18, 2023
XBB.1.16 (Arcturus)
XBB.1.16 is the second variant to earn a nickname under the new system using astronomical names. Both Hyperion (XBB.1.9.1) and Arcturus are names of stars.
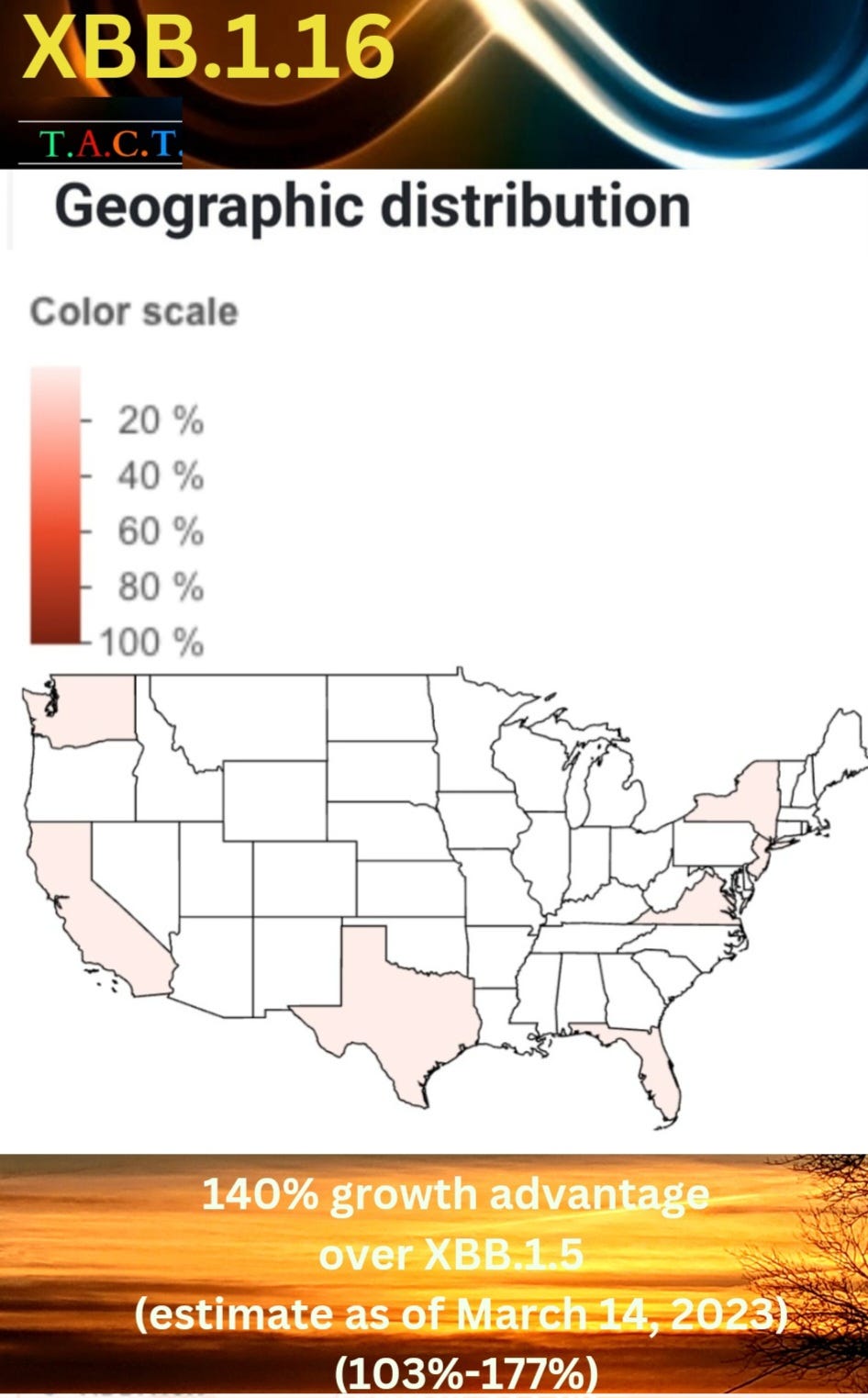
XBB.1.16 has a 140% growth advantage over XBB.1.5 (103.21%-177.41%) as of March 16, 2023. This is an estimate, but the range is tightening as more cases are coming in. This is looking to have a 103% growth advantage at the low end, and at that, it is far more aggressive than XBB.1.5. There are over 20 sub-variants of XBB.1.5 in the U.S., but none of them have this advantage at this point, making XBB.1.16 a variant we will have to watch very closely.
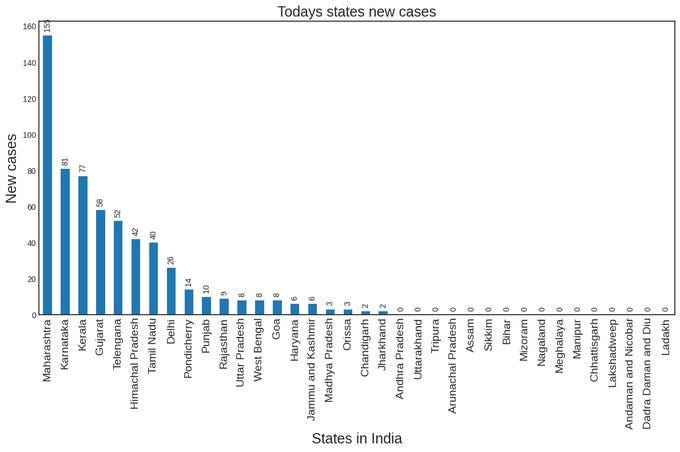
XBB1.16, which is thought to have started in the Indian state of Maharashtra, is now causing a new wave of COVID infections in Telangana, Maharashtra, Gujarat, Kerala, and Karnataka. India is reporting over 800 new daily cases for the first time in 126 days while the number of active cases climbed to 5,389, according to Union health ministry data.
In India, Union Health Secretary Rajesh Bhushan has said in a letter sent to six states, Maharashtra, Gujarat, Telangana, Tamil Nadu, Kerala and Karnataka, to follow a risk assessment-based approach to prevent and contain the spread of COVID-19 infections. He said to examine the situation at a micro-level and maintain focus on the implementation of necessary measures for prompt and effective management of the disease, ensuring compliance with various health advisories issued by the Ministry of Health.
“It is essential that the state must maintain a strict watch and take preemptive action if required in any areas of concern to control emerging spread of infection,” Bhushan wrote.
At the same time there is an increasing number of Influenza cases caused by the H3N2 subtype. Keep in mind that coinfections of COVID, the flu and other viruses are often more severe. On top of that, this report posted on March 15, 2023, says there is a mutated adenovirus that is hospitalizing and killing children.
“Virus outbreak in West Bengal leaves 19 children dead and thousands in hospital”
”More than 3,000 children have been admitted to hospitals with severe flu-like symptoms” (India)
“The Association of Health Service Doctors warns that the numbers of deaths and cases are likely to be much higher than official statistics. Local media reports put the death toll at more than 100 children. The state’s healthcare system is struggling to cope. Some hospitals have said their pediatric wards are full, and there are reports of children having to share hospital beds. The “officials” are reporting that this is caused by “two mutated adenovirus strains.”
They say that this adenovirus is also found in Maharashtra and Karnataka. Coincidentally, the same states have rapidly increasing cases of XBB.1.16. It is likely that adenovirus and influenza are taking advantage of suppressed immune systems during and following infections with the XBB.1.5 variants and now the XBB.1.16 variant.
We already have studies showing how COVID can infect CD-4 T-cells, dendritic cells, and neutrophils. We know that COVID has been listed as one of the leading causes of Lymphocytopenia. A peer-reviewed study, "Immune cell dysregulation is a driver of COVID severity," noted a "decreased total conventional DC (cDC), conventional type 2 DC (DC2), and plasmacytoid DC (pDC). CyTOF also showed lymphopenia of CD4 and CD8 T cell populations."
COVID impacts everyone’s immune system to varying degrees. Some people recover faster than others, but it can take weeks to more than eight months to recover.
Recombinant variants of SARS-CoV-2 like XBB are generated when two or more variants infect a cell at the same time. To better understand this we broke COVID’s life strategy down into 5 steps. To read more about the 5 steps and how reinfections with these variants may cause significant damage to younger children’s immune system follow the link below.
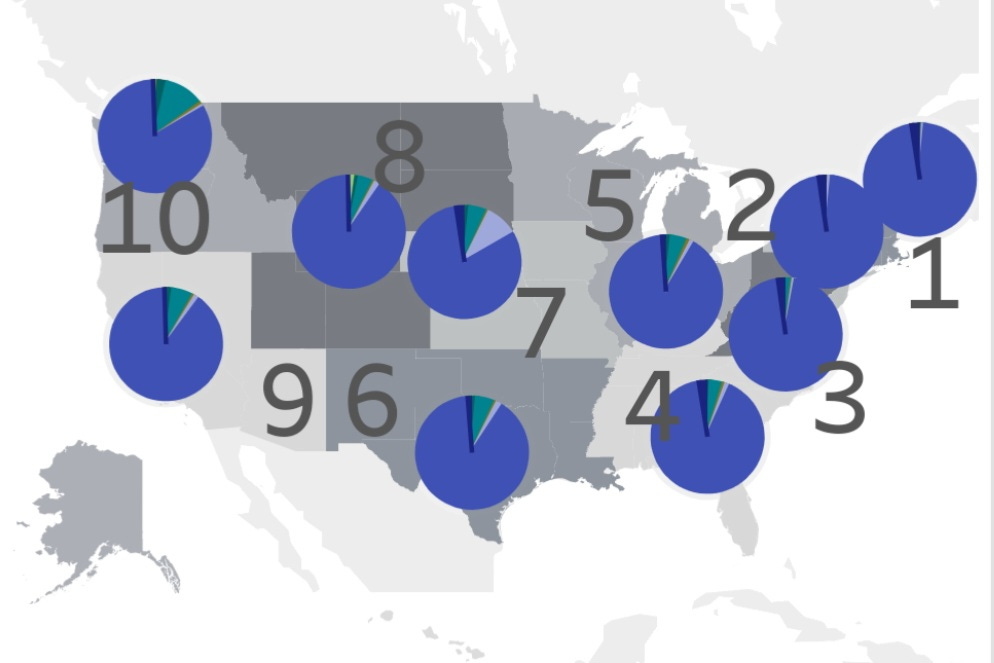
Below, we can see that XBB.1.16 has already landed in the U.S. and is spreading in multiple states.
The U.K. Health Security Agency, in technical briefing 51, described XBB.1.16 as a variant they are monitoring. They point out that XBB.1.16 is a lineage with “3 additional spike mutations (E180V, K478R, and S486P)".
Genomic Sequencing shows that XBB.1.16 has already infiltrated many areas in the U.S. and the U.K.
The U.K. Health Security Agency didn’t mention that XBB.1.16 also has ORF9b:I5T and ORF9b:N55S mutations.
Why are mutations in ORF9b particularly concerning?
This study concludes that their findings “unveil the innate immunosuppression by ORF9b.”
A study published in May 2021 says, “The results showed that SARS-CoV-2 ORF9b negatively regulates antiviral immunity and thus facilitates viral replication.”
How ORF9B and the N-Protein Help COVID Suppress the Immune Response
Note: This section gets a bit more technical so if you don’t have time to digest this then skip down to U.K. Variant Proportions
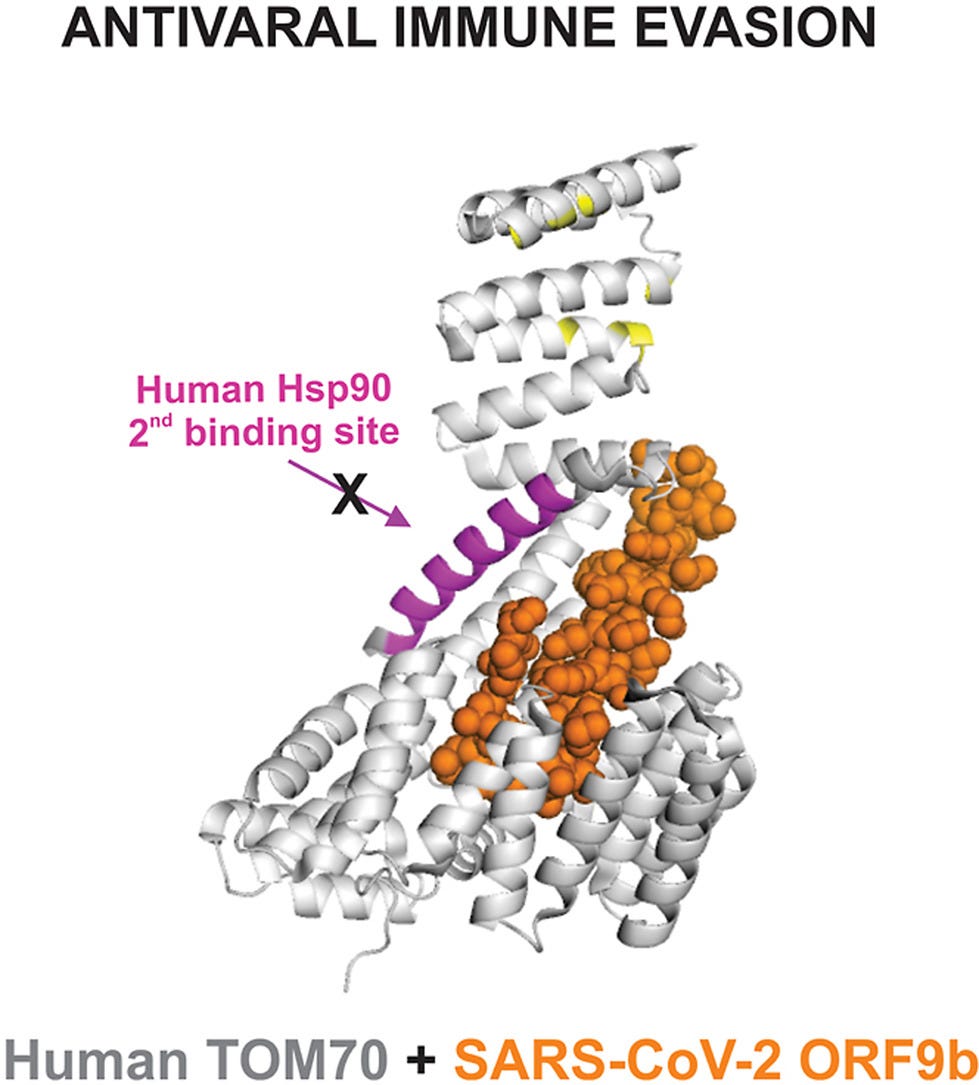
Published in Sept 2022, this study describes, “Accessory proteins of SARS-CoV-2 are encoded by individual ORFs, such as ORF9b, which is involved in modulating the response to infection. This response triggers a cascade that leads to the production of the antiviral protein interferon and includes the mitochondrial antiviral signaling (MAVS) protein. ORF9b likely disrupts the response by binding to the mitochondrial outer membrane protein TOM70.”
“Importantly, protein–protein interaction assays demonstrated that full-length human Hsp90 is capable of binding to free TOM70 but not to the ORF9b-TOM70 complex. To narrow down the nature of this inhibition, the isolated C-terminal domain of Hsp90 was also tested. These results were used to build a model of the mechanism of inhibition, in which ORF9b efficiently targets two sites of interaction between TOM70 and Hsp90. The findings showed that ORF9b complexed with TOM70 prevents the interaction with Hsp90, and this is one major explanation for SARS-CoV-2 evasion of host innate immunity via the inhibition of the interferon activation pathway.”
A study published on February 18, 2023 has a great technical definition of SARS-Cov-2 and in particular the function of the N-protein. If you don’t have time to digest this then skip to the next section.
“SARS-CoV-2 is the third highly-pathogenic human coronavirus in the past two decades, the first two being SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV). SARS-CoV-2 is an enveloped positive-sense single-stranded RNA (+ssRNA) virus belonging to the genus β-coronavirus in the family Coronaviridae. SARS-CoV-2 possesses a genome of approximately 30 kb in size. The 5′-terminal two-thirds of its genome encodes two overlapping polyproteins, pp1a and pp1ab. These polyproteins are further digested by two viral proteases to generate 16 individual non-structural proteins (Nsps) in total, which are essential for the viral replication/transcription complex (RTC) formation. While the 3′-proximal one-third of the SARS-CoV-2 genome encodes a set of accessory proteins and four structural proteins (spike (S), envelope (E), membrane (M), and nucleocapsid (N)).”
“CoV-N protein is a multifunctional protein that plays a crucial role in the viral life cycle. It exhibits a highly conserved architecture in all four genera of coronaviruses. The N protein comprises the N-terminal domain (N-NTD) and the C-terminal domain (N-CTD), sandwiched by three intrinsically disordered regions (IDRs), namely the N-terminal arm (N-arm), the central linker region (LKR) containing the Ser/Arg (SR)-rich motif, and the C-terminal tail (C-tail). The fundamental function of the N protein is to interact with viral genomic RNA to form a helical ribonucleoprotein (RNP) complex, which facilitates viral transcription and assembly. Also, the N protein was identified to assist viral replication and infectivity through liquid-liquid phase separation (LLPS) in SARS-CoV-2. LLPS is mediated by its IDRs and RNA binding. Furthermore, the N protein displays extensive protein-protein interactions (PPIs) with host and viral proteins. For example, the N protein can bind several components (such as G3BP-1/2 (GTPase-activating protein SH3 domain-binding protein 1/2) and hnRNPs (heterogeneous nuclear ribonucleoproteins)) of host stress granules to induce LLPS. It can also interact with host NLRP3 (NLR family PYRIN domain containing-3) to prompt hyperinflammation. Meanwhile, N protein shows the ability to interact with viral M protein for viral assembly. In particular, the N protein interacts with Nsp3 to facilitate viral replication and infectivity”
U.K. Variant Proportions
The U.K. Health Security Agency, in technical briefing 51, posted the variant proportions update that shows data through March 6, 2023. It shows XBB.1.5 continuing to expand, squeezing out the other variants. The question is if XBB.1.16 or a similar subvariant will change this in the near future and if so what impacts will it have on health.
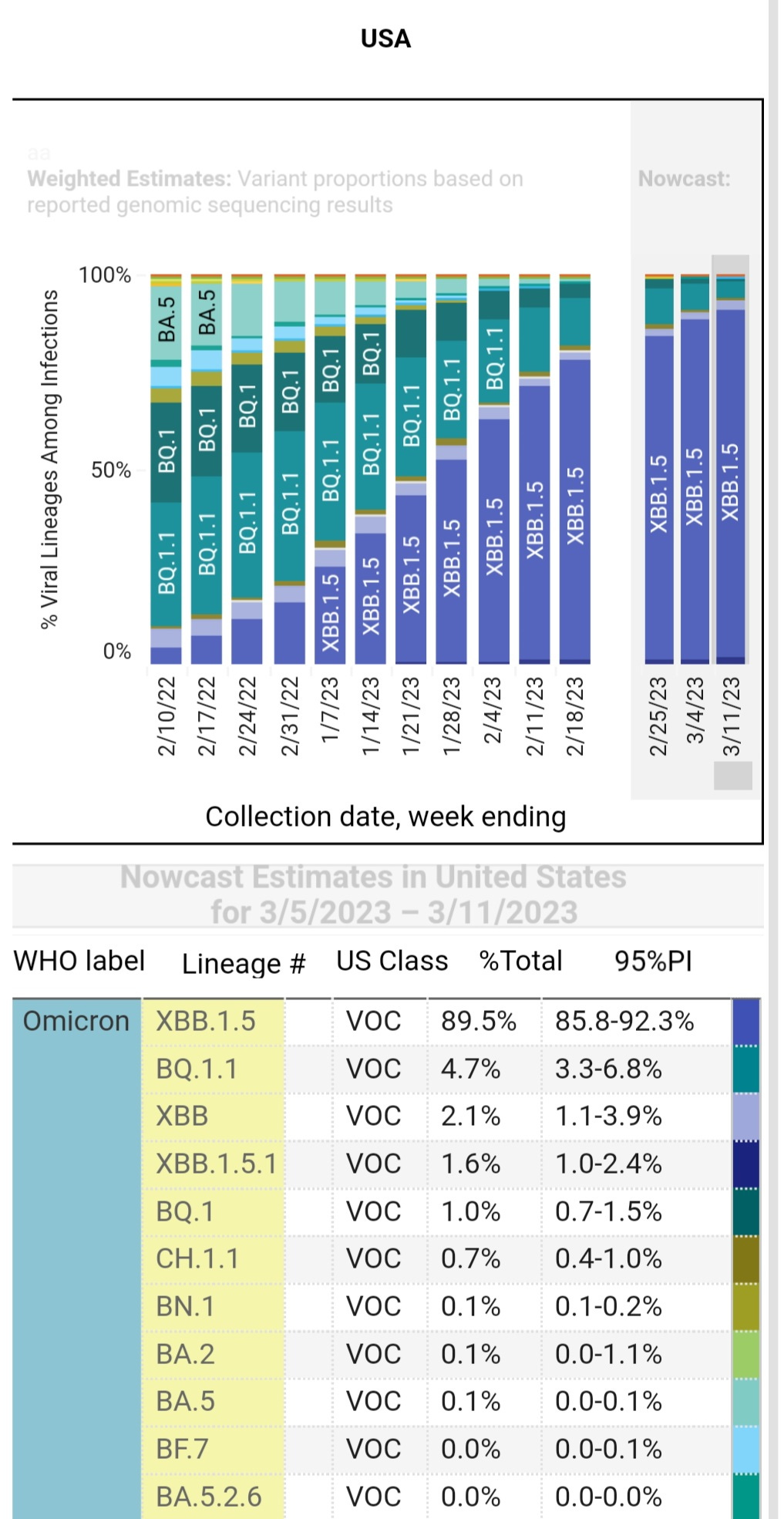
U.S. Variant Proportions
The XBB 1.5 has been the dominant lineage of COVID in India and the U.S. The latest update shows that XBB.1.5 and its subvariants make up almost 90% of new cases nationally. Let’s hope that the CDC updates its graphs as they get the data instead of delaying weeks like they did with the XBB.1.5.
Canada Variant Proportions
Canada’s variant proportions are almost a month behind but as of February 19th, XBB.1.5 continues to expand its presence.
School age children are being infected with XBB.1.16 more than other age groups according to the sequencing data. Coincidentally, we have the report of all the children hospitalized in India with some confirmed but most “suspected” with having adenovirus or flu.
XBB.1.5 has been widespread in India, much like it has been in the U.S., Canada, the U.K. and around the world. India is having outbreaks of a mutated adenovirus, influenza, and a new highly transmissible and immune suppressive variant, XBB.1.16. Together these viruses are putting 1000s of children in the hospital and killing up to 100 already. Shouldn’t we be ramping up protections for children in the U.S., Canada, the U.K., Germany, Australia, South Africa, and everywhere else around the world?
We must press our leaders to invest in the health of children and our communities by installing new or updating existing ventilation and filtration in schools to filter out 99% of pathogens and to have an air exchange rate capable of maintaining CO2 at 600 ppm. This is an attainable goal using existing technology. This will significantly limit in school transmission of not only viruses, but also bacteria, mold, allergens, and other airborne pathogens. This is one investment that can protect generations of children, enhancing the health of everyone, while bringing down the incredible costs of healthcare now and into the future.
We have an opportunity to use the cumulative science, data, and technology to provide a healthier future for our children and future generations, but we must invest the resources necessary to make this happen. This won’t happen without advocacy.
T.A.C.T. is reporting the latest science, data, and analysis in order to achieve a sustainable path forward.
Let us know what you're seeing or experiencing where you are or any other insights you may have.