COVID Variant Update: LP.8.1 Dominates for Now—But the Next Variant Wave Is Building. A Deep Dive into NB.1.8.1
NB.1.8.1 vs XFG: Which Variant Has the Edge? A Long COVID Update from the W.H.O.
As of this week, LP.8.1 now accounts for 64% of all COVID-19 cases in the U.S. This marks a major shift in the landscape of SARS-CoV-2—one that TACT accurately predicted back on January 11, 2024, when XEC was still the dominant variant. At the time, we warned that LP.8.1’s rapid global growth and unique genetic makeup signaled its potential to outcompete others. That prediction has now come to pass.
What makes LP.8.1 so successful? It carries notable spike protein mutations, including V445R, which helps the virus bind more tightly to human cells—particularly lung cells. A study published in March 2025 found that “LP.8.1 exhibits exceptionally high ACE2 binding as well as high immune evasion, similar to XEC.” These features likely contributed to LP.8.1’s rise and sustained dominance.
The World Health Organization (WHO) designated LP.8.1 as a variant under monitoring in early February. Though it’s likely to cause a spike in cases over spring break and the Easter holiday, most people with recent vaccinations or prior infections still have strong protection against severe disease.
NB.1.8.1, XFG, and XEC.25.1: Emerging Threats with Advanced Immune Escape
Now we have other variants gaining momentum—fast. While several new variants are competing in this evolutionary race, one has clearly pulled ahead, showing signs of a significant growth advantage. It’s not yet reflected in CDC variant proportion reports, but in some regions, it surged past 60% of cases even while LP.8.1 was dominant. This variant is now spreading across Southeast Asia, Australia, and has started appearing in the U.S.. and other countries around the world. The full breakdown—and what it means for the next wave—follows further down in the subscriber section below.
TACT’s Article from January 11th on LP.8.1
The CDC Variant Proportions Through April 12, 2025
We can see just how much LP.8.1 has expanded across the U.S. over the past four months, becoming the most common variant in the U.S. continuing to displace all earlier variants.
LP.8.1 is likely taking a serious toll on millions of people—many of whom had no idea they were even infected. A significant portion of cases are asymptomatic, yet tens of thousands are being hospitalized, and thousands have died.
Between January 6 and February 2, 2025, the World Health Organization reported 4,500 COVID-related deaths—but that figure came from just 23 countries (only 10%), and even those reports are incomplete. Meanwhile, hundreds of thousands of people have fallen seriously ill without realizing it was caused by a new COVID variant—often because they never got tested.
Why does this matter?
If you don’t have proof of infection, it becomes much harder—for you or your doctor—to connect new, lingering symptoms to COVID. That matters when Long COVID (also called Post-COVID-19 Condition or PCC) begins to appear in the weeks or months ahead.
Your partner, spouse, or children may start experiencing mental health changes, brain fog, or other unexplained symptoms. Without awareness of the infection, these issues may go unrecognized or misdiagnosed.
Understanding the neurological and psychological impact of Long COVID is crucial—not just for care, but for credibility and treatment.
Long COVID Update from the W.H.O.
Post-COVID-19 Condition (PCC), also known as Long COVID, remains a major public health challenge. While it's difficult to estimate exact rates, but according to the March 2025 W.H.O. COVID update, about 6% of people with symptomatic COVID-19 go on to develop Long COVID symptoms.
The vast majority of Long COVID cases—over 90%—actually follow mild infections. And yes, children are developing Long COVID too. You can see this for yourself at the Long COVID Kids website, here.
If even 6% of children develop Long COVID—and with schools being high-risk environments for transmission—the implications are staggering. We're looking at a generational health crisis unless we take real action to stop the spread of COVID and other airborne diseases in schools.
But there's another path.
If we invest now in clean air and healthy school environments, the benefits will stretch far beyond the pandemic. Improved air quality has been shown to enhance cognitive performance, reduce respiratory illness, and even slow down early aging processes. The result? Healthier, longer lives and a stronger society overall.
When to Expect the Next Wave. New Variants to Watch and Their Possible Consequences
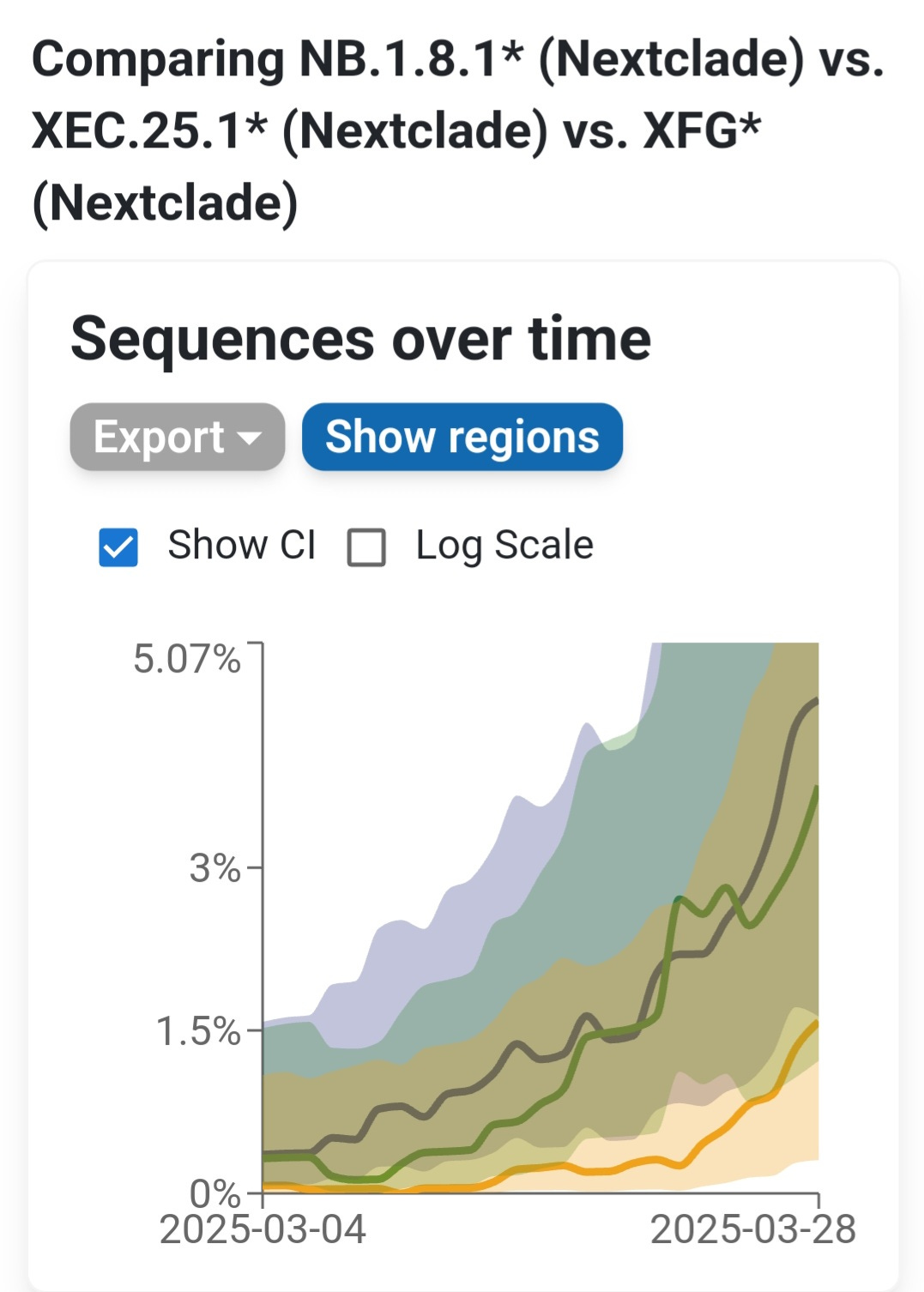
The fastest-moving COVID-19 variant on the planet right now isn’t LP.8.1—it’s NB.1.8.1. This newly designated variant is spreading rapidly across multiple countries, driven by a powerful combination of mutations that give it a distinct evolutionary edge. It’s not just gaining ground—it’s doing so faster than any other variant currently in circulation.
While NB.1.8.1 leads in growth, XFG and XEC.25.1 are other rising contenders. XFG is a recombinant of two successful Pirola sublineages. Both NB.1.8.1 and XFG variants share some key immune-evasive mutations, but they arrived there through very different evolutionary paths. In the section below, we’ll compare how these two variants stack up.
In the subscriber section below, we’ll unpack exactly what makes NB.1.8.1 so formidable, how it evolved, and why it may soon outcompete all current variants. We’ll break down the critical mutations, compare it with XFG and NB.1.8. We’ll examine the real-world implications for vaccines, reinfections, and public health policy.
Emerging Variant NB.1.8 and NB.1.8.1 – Global Spread and Immune Evasion
Rapid Spread in Asia and Growing Presence in South Korea and Australia
Dominance in Hong Kong:
The NB.1.8 lineage—particularly its sub-variant NB.1.8.1—has demonstrated a striking growth advantage in East Asia. In Hong Kong, NB.1.8.1 surged from virtually zero to nearly 90% of all sequenced SARS-CoV-2 cases in just two months.
According to Cov-Spectrum data, it is estimated to have a ~120% weekly growth advantage over other circulating variants, allowing it to rapidly overtake the previously dominant LP.8.1 lineage. By late March 2025, NB.1.8.1 had clearly established itself as the dominant strain in Hong Kong.
Emergence in China, South Korea and Australia:
Beyond Hong Kong, NB.1.8.1 is beginning to take hold in other parts of Asia and Oceania. South Korea has detected NB.1.8.1 in roughly 10% of recent samples, and Western Australia reports it in approximately 5% of sequences. While these levels remain modest, the variant’s foothold across multiple countries suggests early stages of regional expansion. In Southeast Asia, other variants—such as XEC.25.1 and certain LF.7 sublineages—have dominated recent waves (e.g., in Singapore), but NB.1.8.1's presence there and in the U.S. signals potential for broader global spread. That said, NB.1.8.1 and its parent lineage still remain a small minority outside of East Asia and have not yet been reflected in CDC’s variant proportion reports.
Growth Trends and Outlook:
With an estimated 120% weekly growth advantage, NB.1.8.1 is now a leading candidate to drive the next global COVID-19 wave. Its explosive trajectory in Hong Kong is similar to that of previous high-impact variants which rapidly displaced competitors in highly immune populations.
NB.1.8.1 has now been sequenced in Austalia, Canada, China, Finland, France, Hong Kong, Singapore, South Korea, Taiwan, Thailand, the United Kingdom, and the United States, showing how fast it is spreading around the world. In large part this is due to the prevelance of air travel.
Although NB.1.8.1 is not yet classified as a Variant of Interest (VOI) or Variant of Concern (VOC) by international health authorities, its ability to spread rapidly in populations with prior immunity makes it a variant worth watching closely. TACT will continue to monitor its evolution and global trajectory as it gains ground in new regions.
Spike Protein Mutations and Immune Escape
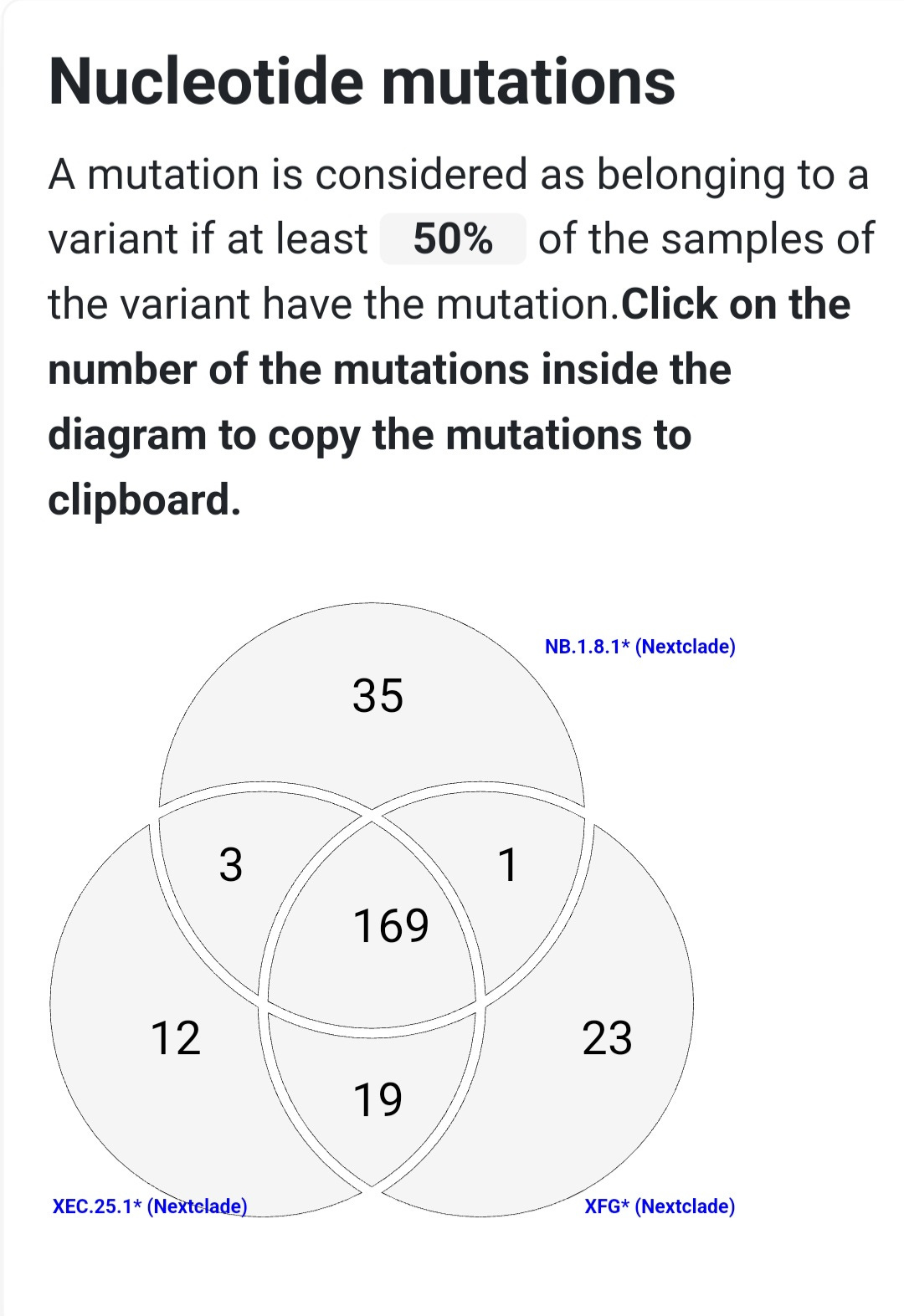
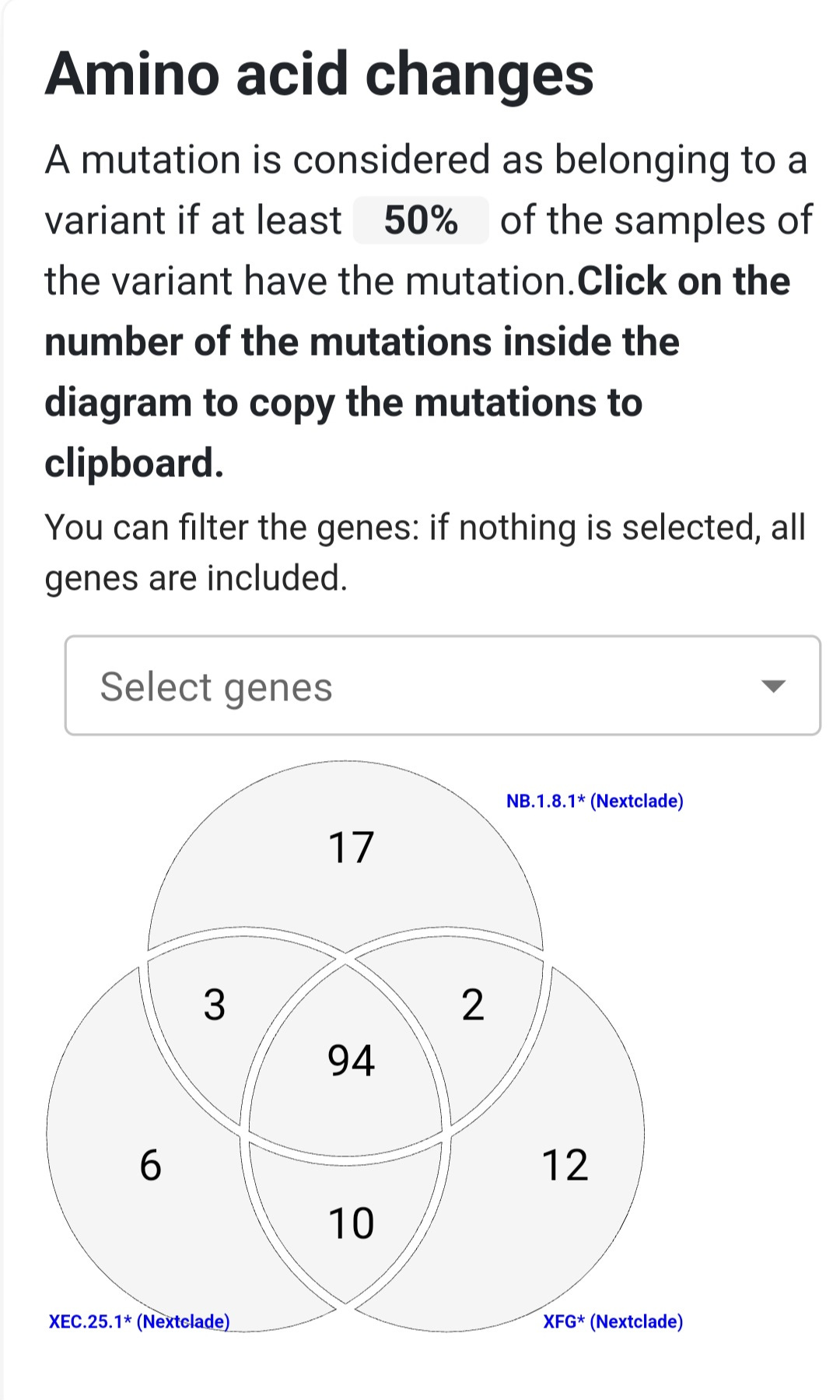
NB.1.8 and its descendant NB.1.8.1 are closely related Omicron sublineages that carry a set of spike protein mutations giving them a significant immune escape advantage. While both share most of these changes, NB.1.8.1 differs by one additional mutation—A435S—which may enhance its ability to evade neutralizing antibodies.
Here are the key spike mutations found in these variants and their likely effects:
All of these mutations are present in NB.1.8.1, while NB.1.8 carries all except A435S, which was acquired later in its evolution.
Taken together, these mutations form a potent escape profile. Remarkably, NB.1.8.1 developed a spike mutation pattern nearly identical to that of XEC.25.1, another highly immune-evasive Omicron subvariant, but did so through a separate evolutionary path. This pattern—called convergent evolution—shows how different lineages under similar immune pressure independently acquire the same advantageous mutations.
NB.1.8.1’s spike evolved step by step, first acquiring F456L, followed by G184S and K478I, then T22N and F59S, Q493E, and finally A435S. This accumulation reflects a strategic adaptation under immune pressure, allowing the variant to slip past many neutralizing antibodies that had been effective against earlier Omicron strains.
As of now, NB.1.8.1 is one of the most immune-evasive variants in circulation, and its continued spread could challenge the effectiveness of existing vaccines and antibody-based therapies.
Virological Features and Impact on Immunity
Immune Evasion as the Primary Driver:
NB.1.8.1’s rapid rise is largely due to its exceptional ability to evade the immune system. Each of its key spike protein mutations weakens a specific layer of antibody protection. For example, F456L alone disables a large portion of neutralizing antibodies generated from prior Omicron infections, while Q493E and A435S knock out additional antibody classes. Collectively, these changes enable NB.1.8.1 to evade most neutralizing antibodies induced by vaccines or earlier infections. Its spike profile includes all five major RBD mutations found in the immune-evasive XEC.25.1 variant—T22N, F59S, F456L, Q493E, A435S—plus two unique additions (G184S and K478I), giving it coverage across a broad range of neutralizing antibody targets. This wide immune escape profile likely explains its ~90% weekly growth advantage in Hong Kong, suggesting immune evasion—rather than significantly increased transmissibility—is driving its spread.
Transmissibility and Viral Fitness:
There’s no current evidence that NB.1.8.1 causes higher viral loads or is inherently more transmissible to people without prior immunity. Its advantage seems to lie in its ability to reinfect individuals who have already had COVID or been vaccinated, including those recently infected with LP.8.1. Some mutations, like F456L and Q493E, occur at the ACE2 binding site and might slightly affect viral entry. However, any loss in binding efficiency appears to be compensated by other mutations—or simply outweighed by the gain in immune escape. The variant’s rapid expansion in Hong Kong confirms that despite its many spike alterations, it remains fit enough to spread efficiently in the real world.
Impact on Vaccines and Antibody Treatments:
NB.1.8.1’s extensive spike changes raise concerns about the reduced effectiveness of monoclonal antibody therapies, most of which have already lost potency against Omicron variants. With mutations at positions 478 and 493, NB.1.8.1 is almost certainly resistant to older antibody cocktails. Compared to current vaccine targets:
NB.1.8.1 carries mutations such as T22N, F59S, G184S, F456L, K478I, Q493E, and A435S
KP.2 shares some overlap but lacks G184S and K478I
JN.1 includes F456L but lacks most of the others
LP.8.1 has its own set (e.g., K679R) but misses nearly all of NB.1.8.1’s key escape mutations
Current boosters based on KP.2 or JN.1 are still expected to offer protection against severe illness, largely due to T-cell responses that target more stable parts of the virus. However, antibody protection against infection is likely reduced, making reinfections more common—even without symptoms. These reinfections still have a cost: each one depletes our finite pool of T-cells and contributes to cell turnover and immune aging, accelerating biological wear and tear over time. See COVID Accelerated Aging for a deeper understanding on this.
Evolutionary Advancement Without Increased Severity:
NB.1.8.1 represents a significant step forward in SARS-CoV-2’s evolution, marked by a carefully accumulated set of mutations that enhance its ability to bypass the immune system. That said, there is no current indication that this variant causes more severe illness than previous Omicron-lineage variants. Its threat lies not in virulence but in reinfection, erosion of immune protection, and the slow, cumulative burden it places on public health and individual health over time.
NB.1.8.1 vs XFG: Which Variant Has the Edge?
NB.1.8.1 and XFG are two emerging descendants of the BA.2.86 (Pirola) COVID-19 variant, each showing strong potential for immune escape.
NB.1.8.1 evolved gradually from earlier Pirola subvariants and has rapidly spread in places like Hong Kong, where it became dominant in just weeks. XFG, on the other hand, is a recombinant—meaning it formed by combining genetic segments from two different variants (LF.7 and LP.8.1.2), and although found on multiple continents, the number sequences being found are few and far between. Both descend from the highly mutated BA.2.86 family, but NB.1.8.1 is showing a clear rapid expansion, that hasn’t been seen since some earlier impactful variants.
Both variants share many spike protein mutations known to help the virus evade immunity, but each also has unique changes that set it apart.
NB.1.8.1 features key mutations like A435S and K478I that may allow it to escape many neutralizing antibodies. XFG, by contrast, pulls together different immune-evasive mutations from its two parent lineages, including H445R and N487D. These differences show that while both variants are adapting to escape immunity, they’ve taken different evolutionary paths to do so.
Outside the spike protein, their genomes are largely similar, with no major changes that would significantly affect viral replication or T-cell immune response.
Neither variant has acquired known mutations that would allow it to fully evade T-cell immunity, which plays a key role in preventing severe illness. However, the heavy concentration of spike mutations in both makes them less responsive to current vaccines and antibody-based treatments, raising the risk of reinfection and reduced vaccine effectiveness.
NB.1.8.1’s rapid rise suggests it may lead the next global wave of infections. NB.1.8.1 appears to combine Omicron-level intrinsic transmissibility with an antigenic profile so different that population immunity offers little hindrance – a recipe for rapid spread. There is no clear sign yet of exponential growth for XFG, whereas NB.1.8.1’s growth is evident. It’s possible that NB.1.8.1’s particular combination (including A435S) gave it an edge that XFG doesn’t yet have. Indeed, analysts are watching if XFG or related recombinants will gain additional Spike changes (e.g. XFG still has 487D rather than 487N seen in NB’s branch – a future mutation at 487 or elsewhere could occur).
Although XFG hasn’t yet taken off, its unique combination of mutations makes it a potential contender if it gains any new mutations. Both variants reinforce the need for continued global surveillance, updated vaccines, and investment in clean air and mitigation strategies. As the virus continues to evolve through both mutation and recombination, understanding these changes is key to staying ahead of the pandemic.
TACT’s Outlook
NB.1.8.1 and XFG represent two of the most advanced evolutionary developments in SARS-CoV-2 this year—NB.1.8.1 through stepwise, mutation-driven adaptation, and XFG through strategic recombination. These variants highlight the virus's ability to continue evolving in both speed and sophistication. Tracking their spread and studying their mutations will be critical in anticipating the next phase of the pandemic. At TACT, we’re following these developments closely.
NB.1.8.1’s explosive rise in Hong Kong is a stark reminder that COVID-19 can still ignite widespread outbreaks—even in populations with high levels of vaccination or prior infection. If NB.1.8.1 spreads more broadly, it could challenge current vaccines and natural immunity, particularly in regions where public health surveillance is weakening. While it hasn't yet been designated a Variant of Concern, its presence in South Korea and Australia signals the potential for global spread through international travel.
These variants are not necessarily more severe, but their enhanced ability to bypass immune defenses is driving reinfections—and possibly the next wave. Hopefully, scientists are evaluating how well existing vaccines and treatments hold up against NB.1.8.1. Though current boosters and antivirals may still protect against serious illness, rising transmission during the summer months last year—historically a quieter period—raises concerns. Just like last year, another late-summer surge appears increasingly likely.
This isn’t happening in isolation. While SARS-CoV-2 continues to evolve, we’re also facing an expanding measles outbreak across multiple U.S. states and the alarming spread of H5N1 bird flu, which is edging closer to sustained human-to-human transmission. Yet, public health transparency and investment—especially in the U.S.—are in retreat. Surveillance systems are being dismantled at a time when vigilance is most needed.
We know what’s coming, and we know how to prepare. What’s missing is the political will to act. This is the time to contact your elected officials and demand renewed investment in public health infrastructure, clean indoor air, and infectious disease monitoring. These threats are not just foreseeable—they are preventable.
Sources:
Cov-Spectrum variant tracking data and analyses of NB.1.8/NB.1.8.1 frequencies
Expert commentary on NB.1.8.1’s mutation profile and convergent evolutionthreadreaderapp.com science.org
Science Immunology study on JN.1 and related variants’ antibody evasion (F456L effect)pubmed.ncbi.nlm.nih.gov
Public health reports (Hong Kong CHP) and genomic surveillance updates on emerging Omicron sublineages chp.gov.hk.










TACT...Wow...you were on this NB.1.8.1 fast. Nicely done. It definitely looks like trouble. WHERE in the United States has this been found? It will be interesting to follow the progression in that area.