COVID Update: FDA's Choice of JN.1 Variant for Upcoming COVID Vaccines in Question/Persistent Infections and Rapid Evolution
Yes, people can be infected with more than one variant at the same time.
Before discussing the latest study on COVID evolution in people it’s important to highlight that due to the weakened immune response from prior COVID infections and SARS-Cov-2’s rapid evolution, more and more people will have persistent infections and co-infections with more than one variant.
Research has shown that SARS-CoV-2 can persist in various body sites long after acute COVID-19. Autopsy and tissue biopsy studies have detected SARS-CoV-2 RNA and protein in multiple tissue types weeks or even months post-infection. For instance, one study found viral RNA and protein in dozens of tissues, including the brain, up to 230 days after symptom onset. More than half of the cases examined had persistent RNA in lymph nodes, the sciatic nerve, ocular tissue, and multiple regions of the central nervous system, such as the brainstem and olfactory nerve. In one individual who died 230 days after mild COVID-19, viral RNA was found in several brain regions, and subgenomic RNA—a marker of recent viral replication—was detected in tissues up to 99 days post-infection, suggesting ongoing viral replication in non-respiratory tissues.
Additionally, SARS-CoV-2 RNA or protein has been identified in tissues months after the initial illness, despite negative results from standard nasopharyngeal PCR testing and no detection in peripheral blood from the same individuals. This indicates that viral persistence occurs predominantly in tissues. Most human tissues are rich in cells expressing the ACE2 and TMPRSS2 receptors, which the virus uses to enter cells. Similar persistence patterns have been observed with other RNA viruses in some survivors. Immune responses against SARS-CoV-2 RNA and protein, indicating persistence, can be localized to tissue and may not be apparent in blood tests.
Earlier research found that co-infections of more than one variant occur frequently, with an estimated rate of ~0.2–0.6% of the observed infections. That may not seem like a lot at first but this adds up over time.
In a study, published January 15, 2024, titled “Systematic detection of co-infection and intra-host recombination in more than 2 million global SARS-CoV-2 samples” they found, "co-infection was observed in 0.35% of the investigated cases." That’s about 7,000 new people per day with coinfections when there are over 2 million people a day getting infected like that which occurred in the U.S. alone from December through February. In 30 days, that’s 210,000 people that have had new coinfections with more than one variant in just the U.S. These are rough numbers but consider that the numbers grow exponentially as new variants are continuing to reinfect people all around the world. Each wave of new variants is more immune evasive and suppressive than the one before it, making persistent infections more and more likely.
The study published on May 28, 2024, sheds light on the prolonged evolution of COVID-19 within individuals. It reveals that when the virus persists in various tissues throughout the body over an extended period, it undergoes significant genetic changes.
Highlights:
People with long-term COVID-19 infections contribute to the emergence of new variants.
The virus accumulates changes in its proteins that allow it to spread more easily and evade the immune system.
Studying these changes will help us identify new variants before they become widespread.
This study looked at eight people who were sick with COVID-19 for a long time. The researchers wanted to see how the virus changed in these people over time. They found that the virus accumulated many mutations, some of which could make it more transmissible (spread easier) and others that could help it evade the immune system or resist treatments.
This is important because it suggests that people with long-term COVID-19 infections could be a breeding ground for new variants. The researchers recommend that we closely monitor these patients to identify new variants early on. Unfortunately, we aren’t doing this but we need to. The study link and abstract are posted at the bottom of this article.
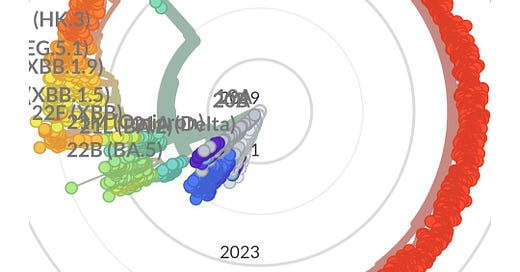
We may not be sequencing people with persistent infections however we do have wastewater data and the level of detailed information that can be attained is surprising. This is highlighted by Marc Jonhsons work tracking variants through wastewater. For example, on June 7, 2024, he posted that he is tracking a highly diverse Delta variant from a sewershed South of Baltimore in the U.S.
He said, “This sewershed only started sequencing earlier this year, so I don't know how long it has been around, but it is clearly Delta-derived, which means the infection probably occurred in the second half of 2021, nearly 3 years ago.”
“The lineage has been showing up pretty consistently in the first sewershed, but on May 21 it also appeared in the sewershed just to the West. Daytrip.” “In recent weeks the lineage has become highly prevalent, sometimes making up over 50% of the signal from a city with over 78 thousand people in it.”
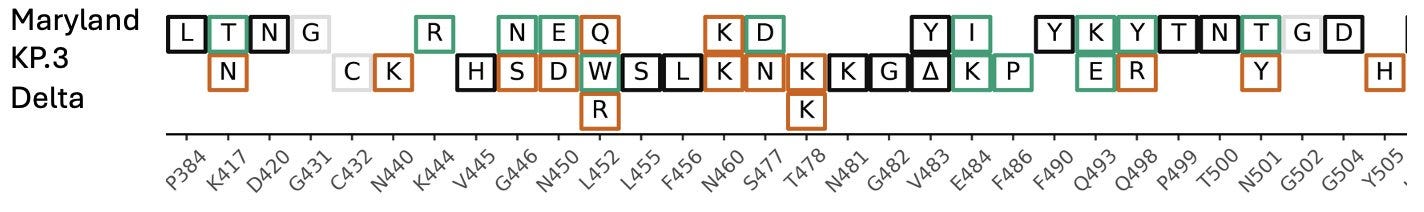
“Because there is so much coverage and so many private mutations (~200), we've been able to reconstruct about 75% of the genome. Here's most of S1 compared with a current JN.1 lineage (KP.3) and Delta.”
“Here's a closeup of the RBD. If this thing were circulating it would look pretty foreign to our immune systems.”
As time progresses, more individuals carrying highly divergent variants of the virus are likely to get infected with new strains, potentially creating completely new recombinant variants that could start spreading. The presence of many people harboring these highly mutated variants significantly increases the odds of more dangerous variants emerging. The rapid pace of viral evolution is alarming, and with limited testing and sequencing, we are largely operating in the dark. Fortunately, over 30 countries are still sequencing cases, but this represents only a small fraction of the total number of cases.
FDA's Choice of JN.1 Variant for Upcoming COVID-19 Vaccines in Question
On June 5, the FDA’s Center for Biologics Evaluation and Research convened with the Vaccines and Related Biological Products Advisory Committee (VRBPAC) to discuss updates to the COVID-19 vaccines for the 2024-2025 period. In a unanimous decision, the committee voted to update the vaccines to target the JN.1 variant.
This decision aligns with a recent move by the European Medicines Agency (EMA), which, following a consultation with the World Health Organization (WHO), also updated their Covid-19 vaccines to target the new strain. The WHO had recommended globally adopting a monovalent JN.1 lineage in future COVID vaccine formulations in an April 26 statement.
However, the decision has sparked some debate. JP Weiland expressed confusion on X (formerly Twitter), stating, “Pfizer's own data from the meeting on booster antigen selection shows a KP.2 type has 100-200% higher titers against the two strongest new lineages than a JN.1 type in a mouse model. Yet the committee still chose JN.1... hard for me to understand this decision.”
Similarly, Yunlong Richard Cao highlighted on X that the Q493E mutation “enables KP.3 to escape a lot of Class 1 V3-53/66 encoded mAbs, even if elicited by JN.1 infection. Since these V3-53/66 NAbs are highly enriched in mRNA vaccine recipients, we would expect KP.3 and KP.2 to show substantial immune evasion even with JN.1 mRNA boosters.”
Supporting these concerns, the study published on April 22, 2024, says “We also observed notable immune evasion of recently emerged JN.1 sublineages, including JN.1+R346T+F456L, with KP.3 showing the most pronounced decrease in neutralization titers by both XBB and JN.1 BTI sera.”
Another study published on May 20, 2024, "Virological characteristics of the SARS-CoV-2 KP.2 variant" shows that KP.2 diminished the XBB.1.5 vaccine efficacy but also points out the fact that it is outcompeting JN.1 which means that in can evade the immune response to the JN.1 variant. "KP.2 shows the most significant resistance to the sera of monovalent XBB.1.5 vaccinee without infection (3.1-fold) as well as those who with infection (1.8-fold). Altogether, these results suggest that the increased immune resistance ability of KP.2 partially contributes to the higher Re more than previous variants including JN.1."
KP.2 and KP.3 and their sub-variants are dominating around the world, each spawning many new variants. For example, the offspring with the greatest growth advantage right now are KP.3.1.1, KP.2.15, KP.3.1.2, and KP.2.3.2. These are JN.1 descendants so thats helpful but they are already highly evolved from JN.1. By the time the updated vaccines are available, the virus will have evolved much further away from JN.1, further reducing the efficacy of the vaccines.
KP.3 variants have been sequenced in Canada the most, followed by the U.K, Australia, the U.S., Japan, and Spain. Again, we are only getting data from a limited number of places and a very limited number of sequences being done in each of them so we are getting a very limited view.
COVID and B-cell Dysregulation
A study published on May 15, 2024, showed how COVID can cause B-cell dysregulation, leading to lowered production of antibodies. “B cells from COVID-19 patients displayed impaired effector functions, evidenced by diminished proliferative capacity, reduced cytokine, and Ab production.” We already know how damaging COVID is to the immune system, and this adds to that evidence. Millions of people with weakened immune systems, not only allow new COVID variants to spread and evolve, it allows for the spread and evolution of other pathogens.
For example, whooping cough, which is caused by the pertussis bacteria, is taking full advantage.

Shaun Lintern reported on June 6, 2024 that in England, “another 3 infants have died from whooping cough, as an extra 1,888 infections were recorded in April alone. Since Jan there have been 4,793 cases, compared to 858 in the entirety of 2023. A total of 8 deaths”
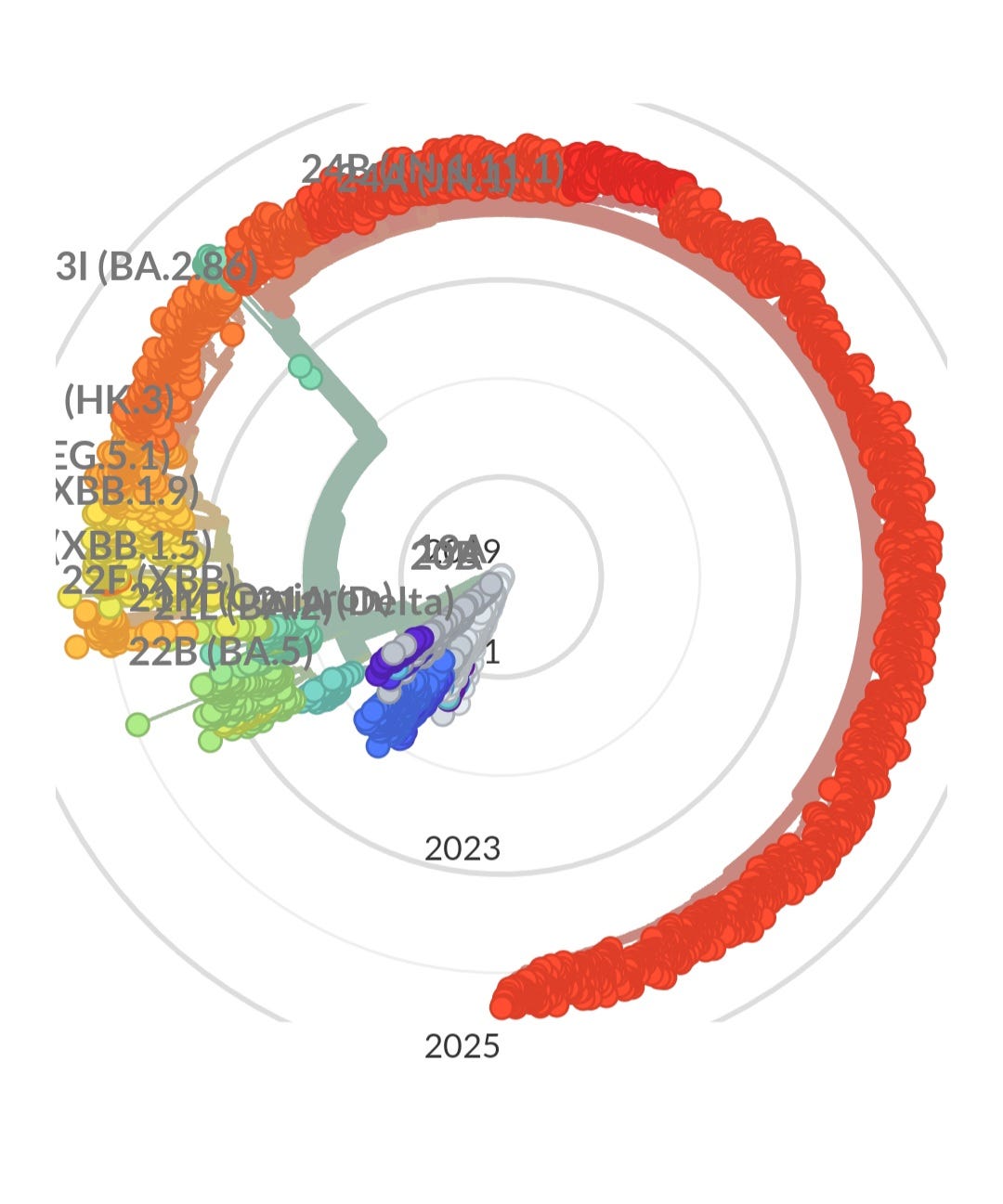
The radial graph below, which represents various sequenced variants over time, illustrates an important trend. During the early stages of the pandemic, when mitigation measures were in place and immunocompromised individuals were protected, the virus evolved minimally. Read “The Silent Sacrifice of the Immunocompromised” to gain a better understanding of how they protected everyone else while society has failed to protect them.
As these protective measures were lifted, the virus has rapidly exploited the increased opportunities for transmission. From late 2023 to today, the evolution of the virus has accelerated dramatically.
Looking ahead to 2025 and beyond, it is evident that the next wave of variants will likely show even more significant evolution, quickly erasing the efficacy of a JN.1 targeted vaccine. Any one of these new variants could result in more severe health outcomes. It's important to recognize that repeated infections, even if asymptomatic, can have serious consequences for individuals and society as a whole.
Coinfections of COVID, Influenza and/or RSV are More Severe
"Prevalence of SARS-CoV-2 and Influenza Coinfection and Clinical Characteristics Among Children and Adolescents Aged 18 Years Who Were Hospitalized or Died with Influenza — United States, 2021–22 Influenza Season": Co-infected children were more likely to require mechanical ventilation (29.4% vs. 13.8%) and had longer hospital stays (median 5 days vs. 3 days) compared to those with influenza alone. https://www.cdc.gov/mmwr/index2023.html
"Viral Coinfection of Children Hospitalized with Severe Acute Respiratory Infections during COVID-19 Pandemic": Children co-infected with hRV, RSV, or bocavirus alongside SARS-CoV-2 had a higher need for oxygen therapy compared to single-virus infections (56.5% vs. 38.5%). The study suggests co-infection might lead to a greater viral load and a more robust inflammatory response, contributing to respiratory distress. https://www.mdpi.com/2227-9032/11/24/3151
"Coinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza virus during the COVID-19 pandemic": Co-infected patients had higher rates of hospitalization (57.1% vs. 38.5%), ICU admission (38.5% vs. 12.9%), and mortality (23.8% vs. 6.5%) compared to single SARS-CoV-2 infection.https://link.springer.com/article/10.1007/s11596-021-2317-2
"COVID-19 bacteremic co-infection is a major risk factor for mortality, ICU admission, and mechanical ventilation": This study found that bacterial co-infections within 48 hours of COVID-19 hospitalization significantly increased the risk of mortality (42.9% vs. 12.9%), ICU admission (64.3% vs. 36.4%), and mechanical ventilation (51.4% vs. 24.3%).https://www.bmj.com/content/368/bmj.m1201
"Severe acute respiratory syndrome coronavirus 2 and respiratory syncytial virus coinfection in children": This retrospective study from the Journal of Medical Virology investigated RSV and SARS-CoV-2 coinfection in children hospitalized with respiratory infections. They found children with coinfection had higher rates of ICU admission, mechanical ventilation, and longer hospital stays compared to those infected with just COVID-19. (Journal of Medical Virology, 2022) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8561020/
"Impact of respiratory syncytial virus and human metapneumovirus co-infections on clinical outcomes in adult COVID-19 patients": This research published in the Journal of Hospital Infection analyzed data from adult COVID-19 patients in Brazil. They observed that co-infected patients with RSV or human metapneumovirus (hMPV) had a higher risk of requiring mechanical ventilation and ICU admission compared to those with sole COVID-19 infection. (Journal of Hospital Infection, 2023) https://academic.oup.com/cid/article-pdf/74/12/2252/44479687/ciab969.pdf
"Viral co-infections in critically ill COVID-19 patients admitted to the intensive care unit: A retrospective observational study": This study from the European Journal of Clinical Microbiology & Infectious Diseases examined viral co-infections in critically ill COVID-19 patients. They found that RSV coinfection was associated with increased mortality risk compared to those without coinfection. (European Journal of Clinical Microbiology & Infectious Diseases, 2023) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8289210/
COVID is Messing With Our Minds
COVID is evolving in a dangerous direction. It has an evolutionary advantage. As long as we allow unmitigated transmission in schools and hospitals to continue, we are aiding its evolution. We can live longer, and we are supposedly smarter, but so far, the virus has retained the upper hand. We must get smarter and take control of this unsustainable situation.
The cumulative cost to individuals, families, and all of society is continuing to grow. An example of this is the brain damage accumulating in society. How is this impacting children's developing minds?
A paper published January 19, 2024, studied 97 people (with an average age of 41 years old who had a mild COVID-19 infection and no history of mental health issues. This was about 79 to 97 days after they were diagnosed. They used interviews, brain scans, and cognitive tests to understand how the virus might affect the brain. They compared them to 77 people who didn't have COVID-19. The brain scans looked at the white matter using diffusion tensor images, and they also studied brain activity while resting. Some patients mentioned problems like memory loss (36%), fatigue (31%), and headaches (29%). The detailed analysis found that many participants had symptoms like fatigue (83%), feeling very sleepy (35%), trouble with certain types of word games (21%), difficulty with verbal memory (16%), and other issues. Will the damage be permanent? If it isn’t permanent, how can people recover if they are reinfected every 6 months to a year? Many people are reinfected much faster than that and with more persistent infections, the cumulative toll is gaining momentum.
Emmanuel, who has been posting excellent breakdowns of the latest studies on X, reported on a study published on May 28, 2024, that explained one of the mechanisms of how COVID-19 influences our brains.
“𝙒𝙝𝙮 𝙨𝙤 𝙢𝙖𝙣𝙮 𝙉𝙀𝙐𝙍𝙊𝙋𝙎𝙔𝘾𝙃𝙄𝘼𝙏𝙍𝙄𝘾 𝙎𝙔𝙈𝙋𝙏𝙊𝙈𝙎 𝙖𝙨𝙨𝙤𝙘𝙞𝙖𝙩𝙚𝙙 𝙬𝙞𝙩𝙝 𝙡𝙤𝙣𝙜-𝘾𝙊𝙑𝙄𝘿? 𝘼𝙣 𝙚𝙭𝙥𝙡𝙖𝙣𝙖𝙩𝙞𝙤𝙣: 𝙩𝙝𝙚 𝙆𝙔𝙉𝙐𝙍𝙀𝙉𝙄𝙉𝙀 𝙋𝘼𝙏𝙃𝙒𝘼𝙔.”
Emmanuel explained, “The SARS-CoV-2 virus that causes COVID-19 can significantly alter levels of metabolites in the kynurenine pathway (KP), which metabolizes the essential amino acid tryptophan. During acute infection, inflammation raises levels of cytokines like IFN-γ.” “Prolonged KP activation may explain long-term issues after COVID-19. Studies found cognitive impairment in post-acute patients linked to high QUIN and 3-HAA over months. Chronic headaches, fatigue and mood changes in long COVID may also involve KP/NMDA receptor interactions.”
Resource
World Health Network Service Provider Database
We need your help to build this database. This is a growing database of COVID-cautious doctors on the World Health Network website. If you know any providers, please add them to the database.
It's important to verify any provider on the database because they may have changed their protocols since it was first input.
How to use the provider database:
https://whn.global/help-provider-database/
Search Providers:
https://whn.global/search-providers/
Here are some of the technical terms explained:
Intra-host evolution: This refers to how the virus changes within a single person over time.
Inter-host evolution: This refers to how the virus changes as it spreads from person to person.
Antigenically divergent variants: These are variants that the immune system has difficulty recognizing.
Amino acid (aa) changes: These are changes in the building blocks of proteins. Proteins are essential for the virus to function.
Spike (S) glycoprotein: This is a protein on the surface of the virus that it uses to enter cells.
Phylogenetic analysis: This is a method used to reconstruct the evolutionary history of a virus.
Study published on May 28, 2024: "Persistence of SARS-CoV-2 infection and viral intra- and inter-host evolution in COVID-19 hospitalized patients"
Abstract: "Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) persistence in COVID-19 patients could play a key role in the emergence of variants of concern. The rapid intra-host evolution of SARS-CoV-2 may result in an increased transmissibility, immune and therapeutic escape which could be a direct consequence of COVID-19 epidemic currents. In this context, a longitudinal retrospective study on eight consecutive COVID-19 patients with persistent SARS-CoV-2 infection, from January 2022 to March 2023, was conducted. To characterize the intra- and inter-host viral evolution, whole genome sequencing and phylogenetic analysis were performed on nasopharyngeal samples collected at different time points. Phylogenetic reconstruction revealed an accelerated SARS-CoV-2 intra-host evolution and emergence of antigenically divergent variants. The Bayesian inference and principal coordinate analysis analysis showed a host-based genomic structuring among antigenically divergent variants, that might reflect the positive effect of containment practices, within the critical hospital area. All longitudinal antigenically divergent isolates shared a wide range of amino acidic (aa) changes, particularly in the Spike (S) glycoprotein, that increased viral transmissibility (K417N, S477N, N501Y and Q498R), enhanced infectivity (R346T, S373P, R408S, T478K, Q498R, Y505H, D614G, H655Y, N679K and P681H), caused host immune escape (S371L, S375F, T376A, K417N, and K444T/R) and displayed partial or complete resistance to treatments (G339D, R346K/T, S371F/L, S375F, T376A, D405N, N440K, G446S, N460K, E484A, F486V, Q493R, G496S and Q498R). These results suggest that multiple novel variants which emerge in the patient during persistent infection, might spread to another individual and continue to evolve. A pro-active genomic surveillance of persistent SARS-CoV-2 infected patients is recommended to identify genetically divergent lineages before their diffusion."
Link to the study: https://onlinelibrary.wiley.com/doi/10.1002/jmv.29708